Review of kava use among Aboriginal and Torres Strait Islander people
ReviewBy Julia Butt1
1 National Drug Research Institute, Curtin University
Introduction
This review provides a comprehensive synthesis of key information on the use of kava among Aboriginal people in Australia. The review begins by summarising how kava is used traditionally, its immediate effects, and its chemistry. This is followed by a summary of the research into the effects of kava on health. The paper will then review the use of kava in Australia; the history of its introduction to Aboriginal people and the impacts of kava on the health and wellbeing of Aboriginal Australians. This discussion is focussed on the use of kava in Arnhem Land (Northern Territory) communities because concerns regarding the prevalence and effects of kava use are limited to this geographic region.
The review critically discusses the regulation of kava in Australia, including the impacts these regulations have had on Aboriginal communities. The paper reviews strategies to reduce kava related harms and concludes by discussing possible future directions for research and action for minimising further harm.
About this review
This review draws mostly on journal publications, government reports, national data collections and national surveys, the majority of which can be accessed through the HealthInfoNet’s Bibliography.
Edith Cowan University prefers to use the term ‘Aboriginal and Torres Strait Islander’ rather than ‘Indigenous Australian’. However, when referencing information from other sources, our authors are ethically bound to utilise the terms from the original source unless they can obtain clarification from the report authors/copyright holders. As a result, readers may see these terms used interchangeably with the term ‘Indigenous’ in some instances. If they have any concerns they are advised to contact the HealthInfoNet for further information.
Acknowledgements
Special thanks are extended to:
- the anonymous reviewer whose comments greatly assisted finalisation of this review
- staff at the Australian Indigenous HealthInfoNet for their assistance and support
- the Australian Government Department of Health for their ongoing support of the work of the Australian Indigenous HealthInfoNet.
Key facts
- Kava is a psychoactive drink made from the roots of the kava plant Piper methysticum Forst F. It has been grown and consumed for over 3,000 years across the Pacific for ceremonial, recreational and medicinal purposes. The prevalence of kava use in Australia is low. It is used by some Pacific Islander Australians; it is also consumed in eight Aboriginal communities in Arnhem Land, Northern Territory.
- The effects of kava intoxication include feelings of sociability, peace, reduced stress and anxiety, and positive mood while retaining clear headedness. Increasing levels of intoxication can lead to feelings such as numb mouth, sedation, a sense of muscle weakness, ataxia, and fatigue, and at very high doses it can lead to increased levels of harm.
- The psychoactive components of kava are kavalactones; they act as central nervous system depressants. Extracts of kavalactones are now used therapeutically in alternative western medicine as a treatment for anxiety and sleep disorders.
- International reviews into kava use have concluded that the harms associated with moderate kava use are low. There is however a lack of empirical evidence in relation to the long term (chronic) effects of high levels of kava use.
- Concerns about the health effects of heavy kava use have been raised in Australia since the late 1980s, and more recently in the Pacific.
- Research to date demonstrates that kava use may cause kava dermopathy, raised liver enzymes, nausea and weight loss in a dose dependent fashion. Kava use has been associated with a range of other negative effects such as poor general health, red eyes, low motivation, seizures and heart disease.
- Kava was introduced to Arnhem Land in 1982, following a cultural exchange between the Yolgnu people and a Fijian community. It was thought that kava may provide an alternative to alcohol, reduce alcohol related harm and facilitate fellowship and social cohesion.
- A considerable amount of research into the use of kava in Arnhem Land communities was conducted between 1987 and 2006; since 2006 there has been no published research.
- Research into the prevalence and pattern of kava use demonstrates that between its introduction and 2002 the proportion of people using kava in kava-using communities increased as did the amount of kava consumed by drinkers. In some studies between 70-80% of males in kava-using communities were kava drinkers. Furthermore kava consumption was in excess of the level at which harms are thought to occur.
- The high prevalence of heavy kava use has led to a decline in community activity, cultural activity, community cleanliness and participation in employment. Research has identified significant economic impacts of kava on communities.
- Kava has a complex regulatory history with six major changes in regulation occurring between 1982 and 2006.
- A demand for kava persisted during all periods of regulation studied; kava was at its lowest availability during 2000 and 2001 (when kava was illegal) and its highest availability when kava was unregulated and during licensing periods.
- Currently kava remains on the prohibited and restricted imports list under the Customs (Prohibited Imports) Regulations 1956 Act. Incoming passengers to Australia may bring two kilograms of kava for personal use. A change to the import restriction has been recently foreshadowed and may see these levels raised.
- There is no published evidence regarding the outcomes of the importation ban. Anecdotal reports suggest that the ongoing effects have been a reduction in overall kava availability and harms, but that heavy kava use is still present, albeit more hidden.
- The process by which the ban was implemented undermined Arnhem Land communities. It demonstrates clearly that collaborative community engagement is required to develop policy and also to implement, evaluate and adapt it.
- Approaches to reduce harmful kava use in Arnhem Land have been largely limited to supply reduction regulations.
- Future directions include the need for ongoing high quality research into the long term effects on kava and health, action and resourcing to prevent kava trafficking, an increase in resourcing to services support people experiencing kava related harms, and collaborative development of and evaluation of kava policy.
Overview of kava
What is kava?
Kava is the term commonly used to describe the plant Piper methysticum Forst F., a member of the pepper family Piperaceae, and for the psychoactive drink made from its roots. Kava’s effects include reducing anxiety, relaxing muscles, sedation and numbing of the mouth [1, 2]. It has been grown and consumed for ceremonial, recreational and medicinal purposes [2] for over 3,000 years across the Pacific. This includes parts of Melanesia (e.g. Vanuatu, Fiji, Solomon Islands), throughout Polynesia (e.g. Tonga, Samoa but excluding New Zealand) and infrequently in Micronesia [3, 4]. Kava crops form a significant part of the economies of many of these nations [5]. While known across the Pacific region as awa, kawa, kava, kava kava, yaqona, grog, tigwa and sakau [6, 7], in Australia it is predominantly known as kava.
Traditionally prepared kava is used by Pacific Islander migrants in a range of countries including the United States of America, New Zealand, and Australia. In Australia, it is used by Pacific Islander (Tongan, Samoan and Fijian including Indo-Fijian communities) in all states and territories. In addition kava is used by a small number of Aboriginal communities in Arnhem Land, Northern Territory (NT). This review is focused on the use of kava by Aboriginal people in Australia – but it is important to recognise from the outset that concerns related to kava use and kava related harms are restricted to eight major communities, and several homeland communities in Arnhem Land. Concerns related to kava use do not extend to other Aboriginal nor Torres Strait Islander communities.
As a traditionally prepared drink, kava is non-fermented and consumed shortly after preparation. It is made from an infusion of chewed, ground, pounded, or otherwise macerated fresh or dried kava root with either cold water or coconut milk. It is typically prepared and shared from a communal bowl and drunk from coconut shells, which are passed around. Historically kava was consumed ‘green’, soon after harvest; it is now more common for it to be dried and marketed as powder [3, 5]. The most commonly consumed form of kava in Australia is dried and powdered root-stock from Fiji and Tonga, prepared using either a teabag type method or by mixing dried powder with water and straining the solution prior to consumption.
In addition to the above, kava is being used increasingly in western natural medicine (therapeutic use) as a treatment for anxiety and sleep disorders [2, 8, 9], and as an ingredient in social and recreational beverages [10]. In the therapeutic use of kava, the psychoactive components of kava roots – kavalactones – are extracted from roots via acetone or ethanol (the traditional method uses water extraction). The traditional preparation method is used in Arnhem Land communities and is the focus of this review. However research into the safety of therapeutic kava contributes to understanding the effects of kava and is presented where relevant.
The kava plant is a perennial shrub, of which there are over 115 known cultivars [11]. It has a natural range and area of cultivation restricted to tropical Pacific Islands [7]. Kava is not known to grow in Australia [11], thus all kava in Australia is imported. Harvesting is labour intensive and guided by traditional knowledge of kavalactone concentration. In any kava plant the kavalactones are most concentrated in the lateral roots (rhizomes, roots and roots stems) of the plant [12] and decrease progressively toward aerial parts of the plant [13]. The aerial parts contain toxins and have not traditionally been used [14]. Only roots from plants over four years old are harvested as younger plants have lower concentrations of kavalactones [14].
Kava intoxication and the chemistry of kava
The taste of the traditionally prepared drink is described as earthy, peppery, acrid and astringent [7]. The immediate (acute) effects have been described by ethnographic and participant observation research; they are described in positive terms including sociability, peace, reduced stress and anxiety, and positive mood while retaining clear headedness [15, 16]. Observations of elevated mood are consistent across descriptive literature and have been replicated in two laboratory-based studies [17, 18]. Other effects include a numb mouth, mild sense of euphoria and some alteration of senses. With increasing intoxication feelings include sedation, a sense of muscle weakness, ataxia, a sense of unreality and fatigue [3, 19-21]. At very high doses it can lead to severe ataxia and sedation, paralysis of the extremities, extra pyramidal movements, deafness, dilated pupils, other ocular abnormalities and deep sleep [3, 5, 22, 23]. Kava intoxication does not include excitability or loss of inhibitions.
When kava is consumed, the psychoactive component of kava – kavalactones – are absorbed through the gastrointestinal tract [24]. This absorption is described as poor and variable [11] and dependent on the context; for example if consumed with food, absorption is reduced. Generally after ingestion it takes 1.8 to 3.0 hours to reach peak serum levels [12]. Kavalactones have a dose-dependent depressant effect on the central nervous system (CNS), consistent with feelings of muscle relaxation, sedation, reduced anxiety, anaesthesia etc., (see Sarris et al. [9] for a review), and affect a range of neurotransmitters [25]. The mechanisms by which kavalactones affect neurotrasmitters are not clearly understood. There are a number of hypotheses that have variable support in the literature. These mechanisms involve either reducing the activity of excitory neurotransmission or increasing the activity of inhibitory neurotransmission [2, 12, 26-29]. See Sarris et al. and Showman et al. for reviews [9, 30].
Kavalactones exist in different concentrations in kava depending on genetic and environmental variations of plants [9, 13, 31, 32] thus potency is difficult to establish. Furthermore 19 different kavalactones have been identified in kava, six of which are the major active constituents [9, 13] and are the most studied of the kavalactones. There is no research to date suggesting how proportions of the different kavalactone impact the effects of kava. Research on the physiological effects of kava have been centred on kavalactones, however Showman et al. [30] caution that the known effects may be underestimated by this unilateral focus. The other components of the dried root stock which make part of the beverage include: fibre, water, sugars, proteins, and minerals [14] including alkaloids and flavokavins. Showman et al. [30] suggest that the alkaloids and flavokavins may also induce physiological responses of cells [2, 30, 33].
The effects of kava on health
The World Health Organization (WHO) and the Food and Agriculture Organization (FAO) both of the United Nations have reviewed the health effects of kava (including both traditional and therapeutic uses) and have concluded that the overall potential of harm from kava is low, with negative events described as lethargy, nausea and headaches [10, 34]. A 2005 report by Food Standards Australia and New Zealand (FSANZ) [11] reached a similar conclusion. The toxicity of kava was investigated by the United States National Toxicology Program [33] in 2004, who concluded that the toxicity of kava is most likely dose dependent but considered low [27, 30, 34]. While the overall harms and toxicity of kava are considered low it is important to acknowledge that there is a lack of empirical evidence from which the reviews have been able to draw on, particularly in relation to the long term (chronic) effects of kava use. Concerns about the health effects of heavy kava use have been raised in Australia since the late 1980s [19, 35], and more recently in the Pacific although less so [16, 36-38] [39].
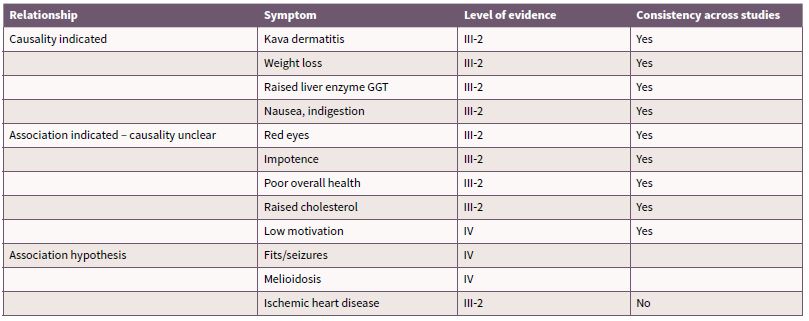
The evidence regarding the specific health effects of kava are predominantly from descriptive studies that have occurred over a short period of time, by a small number of authors. In many of the studies a small cohort of participants are examined on a large range of variables [40-43] which limits the capacity for detailed statistical examination and adherence to methodological rigour. There is a paucity of longitudinal studies and studies using random or matched sampling. There is also more broadly a shortage of epidemiology and public health data in Pacific populations who use kava [30] [44], making it difficult to determine effects of kava which are not contextually and culturally derived. Rychetnik and Madronio [45] conducted a systematic review on empirical studies into health and social effects of traditionally prepared kava (recreational kava use). The review of published research allocated levels of evidence1 based on study design consistent with the levels specified by the National Health and Medical Research Council (NHMRC) [46], and appraised the evidence for each health outcome using standard epidemiological criteria for causality [45]. The outcomes of the review for health effects are summarised in Table 1. The maximum level of evidence is III-2 (from comparative studies with no concurrent controls) which highlights the lack of methodological rigour that has been applied to research into traditionally prepared kava. The review concluded health effects in which causality can be attributed to traditionally prepared kava use are kava dermopathy (skin condition), weight loss, elevated liver enzymes and nausea.
Table 1. Health effects of kava use
Source: Derived from Rychetnik and Madronio (2011) [45]
The section below describes the health effects listed in Table 1 in more detail, as well as a review of the evidence regarding additional hypothesised health effects of kava use. It is worthy to highlight that much of the research into kava-related harms has been from Australia. A far greater proportion of people consume kava in the Pacific yet there has been much less debate about its public health implications. Previous commentators have suggested that this is partly due to traditional practices and limits on kava consumption [15, 35, 47] meaning that it is consumed more moderately in the Pacific. However it is also important to acknowledge that there are growing reports of kava related harm in the region [15, 37, 38], and recreational use (as opposed to ceremonial and medicinal use in which consumption is governed by cultural protocols) is increasing [23, 36-38, 43, 48, 49].
Kava dermopathy
Research in Australia and the Pacific has consistently shown that after prolonged heavy use kava drinkers are likely to experience kava dermopathy – a scaly skin rash (ichthyosiform eruption) or ‘crocodile skin’ [16, 34, 50, 51]. The evidence suggests that there is a dose dependent relationship between kava use and kava dermopathy and that the condition is reversible on reduced consumption or cessation of use [52]. Little is known about the long term consequences of kava dermopathy, its progression or the level of kava use at which it emerges.
Weight loss
Results from research conducted in Arnhem Land have consistently demonstrated an association between kava use, low body mass index (BMI) and weight loss, suggesting that BMI, skin folds and body fat decrease with increasing kava use [40, 42, 53]. This finding has not been demonstrated in any research in the Pacific Islands, however ethnographic accounts describe some ‘heavy’ kava users as ‘lean’ or likely to experience loss of appetite while drinking [16, 37, 52, 54]. However, these descriptions are not quantified and it is argued in the literature that they are exceptional cases. A possible mechanism by which kava may be related to low BMI, as observed in the Arnhem Land research, is that malnutrition may come from loss of time in food preparation, loss of appetite and some kava induced nausea [40, 42].
Liver function
The impact of kava on the liver is the most well studied and controversial of the hypothesised chronic effect of kava use [9, 55-57]. Therapeutic kava products were banned in many European countries between 2002 and 2008 following concern that therapeutic kava use had caused hepatotoxicity and death in several cases (for a more detailed discussion please see [9, 32, 34]). Following these concerns considerable effort was placed into examining the effects of kavalactones and kava products on the liver. Reviews of the cases that had triggered regulatory changes found little concordance between cases and argued that the mechanisms responsible were speculative [57-59]. Consistent with this, the WHO review into kava hepatotoxicity concluded that kavalactones may occasionally cause hepatic adverse reactions and summarised the possible mechanisms; kava-drug interactions, excessive alcohol intake, metabolic immune mediated idiosyncrasy, excessive dose, or pre-existing liver disease [34].
Kava use does affect liver function. In both therapeutic and recreational contexts (in Australia, the US and the Pacific), kava is related to increased levels of the liver enzymes gamma-glutamyl transpeptidase (GGT) and alkaline phosphate (ALP) in a dose dependent fashion [40, 55, 56, 60]. There is some evidence that elevated GGT and ALP returns to normal on cessation of kava use and of note no studies have demonstrated that hepatocellular damage or hepatic failure has occurred as a result of elevated liver enzymes [41, 56]. Thus the clinical significance of raised ALP and GGT is unclear. Only two case studies of liver toxicity among recreational kava users have been reported to date [56]. The possibility that prolonged high levels of kava use may permanently affect liver functioning and liver damage cannot be ruled out [34], however moderate and short term use is unlikely to cause irreversible liver damage.
Nausea
Nausea has been identified as a short term consequence of kava use in both the therapeutic and traditional use literature [23]. Indeed nausea has been identified as the most common side effect from trials of therapeutic kava [34]. Kava is thought to cause nausea through inflammation of the stomach [61].
Ocular effects
In the ethnographic literature kava drinkers are often described as having watery red eyes [16, 20]. Consistent with this observation, Kava [37] reported 72% of ‘very heavy’ users and 57% of ‘heavy’ users in Tonga experienced watery eyes from drinking kava. Alexander et al. [19] reported similar effects in Arnhem Land. In several studies kava has been reported to cause pupil dilation, blurred vision, disturbances of oculomotor equilibrium and photosensitivity [16, 52, 62, 63]. The effect of kava on eyes warrants further research to clarify the incidence, cause and clinical significance of irritated eyes.
Seizures
Seizures have been identified as a serious potential effect of kava use. It has been suggested that heavy kava use may cause seizures as a component of either toxicity or withdrawal, or both. Evidence regarding seizures is limited to one case study [64] and a retrospective review of the medical notes of 21 cases of kava users who had experienced seizures [65]. Kava use was listed as a cause of seizure in approximatley half the cases, however the systematic review notes that the evidence suggests an association between kava and seizures in these cases and that cause has not been proven [45].
Cardiovascular function
There is some evidence which suggests that kava use may have an effect on cardiovascular function. Observational research [40] found heavier kava users had a higher pulse rate, yet this has not been replicated elsewhere. Kava consumption was linked to the deaths of several young sportsmen in Arnhem Land [42], and a subsequent review into sport-related cardiac arrests in Arnhem Land concluded that kava may constitute a risk factor in the exercise context for ischemic heart disease [66]. This has not been studied systematically. The systematic review concluded there was only evidence for an association between ischemic heart disease and kava use (see Table 1) [45].
Poor general health
Self-reported data from both Arnhem Land and the Pacific suggests that people who use kava report lower overall health than those who do not use kava [40]. Furthermore there is some evidence of an association between kava use and melioidosis (which is a common cause of community-acquired pneumonia) [67, 68] and that this may potentially be related to lower immune functioning [42, 69]. One brief study has been conducted to date [42] in which kava users had indicators of lower immune functioning, however the results were not sufficient to conclude that chronic kava use causes a compromised immune system. The mechanism for the relationship between kava use and self-reported poor health is not clear; it may be related to patterns of kava use in which health is neglected and/or immune function is reduced.
‘Amotivational syndrome’ and lethargy
It has been suggested in both the Arnhem Land [31, 70] and Pacific contexts [22, 43] that heavy kava use results in tiredness, lethargy and apathy or a loss of interest in other aspects of life. This group of symptoms is referred to as a kava ‘amotivational’ syndrome. Importantly there is great difficulty in demonstrating an ‘amotivational syndrome’ – it may be an acute ‘hangover’ [15, 71] or a pattern which emerges as an effect of chronic kava use. As an acute consequence ‘amotivation’ is consistent with the soporific and sedating effects of kava, and is also consistent with reports of kava hangovers. In Fijian research investigating patterns of kava use among teachers, participants reported common experiences of hangovers – described by the authors as mental and physical lethargy [15]. These symptoms included reports of feeling sleepy, a lack of energy and disruption of memory recall, with some participants reporting the hangovers could last up to two days [15]. It has not been studied systematically in Australia and warrants further investigation across different cultural settings.
There is little research investigating chronic ‘amotivation’ aside from descriptions in ethnographic and observational research. Concern about heavy kava users neglecting family and community roles is frequently discussed in research and commentary regarding the use of kava in Arnhem Land [21, 31, 72]. It is possible that amotivation is caused by an as yet unidentified mechanism, or perhaps at high levels of habitual use, kava may take priority over other activities resulting in a lack of motivation to attend to other tasks; consistent with characteristics of drug dependence (e.g. [73]).
Cognitive function
The effects of kava on cognitive function are mild. To date the evidence suggests that at low doses (therapeutic doses) and in laboratory settings kava may have a minor positive effect on reaction time and attention with no consistently reported negative effects [17, 18, 74-77]. In a recreational context Cairney, Maruff, et al. [1] compared the performance of kava-intoxicated participants with controls on cognitive tasks. Of note in this study kava-using participants had consumed approximately 200 times the therapeutic kava dose, and had not been consumed kava for the previous 8 hours – although they did present with symptoms of kava intoxication (e.g. ataxia) [78]. Cairney, Maruff et al., reported no difference between the groups on complex cognitive functions [78]. The research demonstrated that intoxicated participants had deficits in visual search tasks (visual attention) as task complexity increased and slowed saccade functioning (rapid small eye movements) which suggest disruption of cerebellar functioning [78]. A more recent brief study conducted in Fiji examined cognitive performance on two tests assessing processing speed by kava users the day following kava use and non-kava users [15]. The results showed that kava-using participants’ performance was significantly lower on a test which measures visual memory, visual motor coordination, psychomotor speed, visual perception and visual scanning. The authors concluded that kava may affect processing speed but not working memory [71]. The authors noted some difference between their findings and that of Cairney et al. [78] however concluded that the consensus suggests kava can cause motor incoordination and visual attention deficits [71].
There is only one study that has investigated the chronic effects of kava use on cognition [79]. This study found no difference between current, ex- and non- kava users in cognitive or saccade functioning suggesting that there was no lasting cognitive impairment from kava use. They concluded that long term kava use has a benign effect on cognitive functioning [79], a conclusion consistent with that of later reviews (e.g.[77]). Consistent with this there is no detail in the ethnographic research to suggest that cognitive impairment was a concern in traditional kava-using communities.
Ataxia, injury and driving
Aaxia (lack of voluntary coordination of muscle movements) and lack of coordination is a well established consequence of kava intoxication [1, 7, 17, 19, 54, 74] which may arise from the muscle relaxant properties of kava [54, 80, 81]. There are concerns that ataxia may predispose individuals to accident or injury, particularly if driving or operating machinery.
The impact of kava use on driving is of particular interest [1, 9]. There are several cases in New Zealand and Southern California in which drivers under the influence of kava have been detained by police due to erratic driving [82, 83]. In Fiji, Wainqolo conducted a population-based case-control study following motor vehicle accidents [84]. They found that kava use was associated with a fourfold increase in the likelihood of crash involvement. These findings are supported by the findings of a double blind laboratory study. Thompson and colleagues [18] found that participants who consumed therapeutic doses of kava were significantly different to placebo; kava participants felt more sedated, more fuzzy headed, less coordinated, more intoxicated and reported their capacity to drive was less than those who had placebo. Thus, the evidence to date suggests that driving under the influence of kava presents a risk for harm [85]. Not surprisingly consumers of therapeutic kava are advised to exercise caution when driving or operating heavy machinery [9, 34].
Kava dependence
Observations of excessive kava use have been documented for centuries across the Pacific in ethnographic research [22, 43] and since the late 1980s in Australia [19, 35]. Despite these observations, few studies have investigated clinical levels of kava use, in particular kava dependence and whether tolerance and withdrawal syndromes exist for kava. With respect to tolerance Duffield and Jamieson [27] found that mice developed tolerance to aqueous kava but these results have not been replicated. d’Abbs and Burns [21] suggested that kava users may become tolerant to some effects of kava but not to others, but their observations have not been tested.
Similarly, withdrawal after abstinence from kava has not been studied satisfactorily [25]. Health workers in Vanuatu have described clinical symptoms (agitation and sleep disturbance) which they understand as kava withdrawal [43] and Clough et al. [65] reported several case studies in which kava withdrawal may have been associated with seizures.
The available evidence does suggest that people consume kava at levels consistent with a drug dependence (symptoms include craving, neglect of roles in the family and community, difficulties controlling kava use, and continued use in the face of persistent negative consequences from use [73]). For example, researchers in the Pacific [42], and Australia [35] describe heavy kava users as having an obsession with kava and prioritising kava over other needs like preparing and eating food. Prevalence of use and risk of dependence has not been investigated and warrants further attention.
Drug interaction effects
As with all drugs, there is a risk that the interaction of kava with other drugs has the potential to cause harms (e.g. [34]). Kava affects the CNS; as such it may act additively with other CNS depressants such as alcohol and benzodiazepines. Indeed alcohol has been found to potentiate kava inebriation in mice [27] and in human participants in laboratory settings [17]. Foo and Lemon examined cognitive performance on participants who had consumed alcohol, kava, or alcohol and kava but did not know which substance they had consumed [17]. They found that kava-consuming participants performed better than alcohol-consuming or alcohol and kava-consuming participants. Participants who consumed both alcohol and kava performed significantly worse than those consuming only alcohol. Thus consuming alcohol with kava may increase the negative cognitive effects of alcohol [25].
Recent research has suggested that kava also may affect how other drugs are metabolised because kavalactones inhibit some enzymes (CYP450) important for drug metabolism [24, 86]. By inhibiting these enzymes levels of other drugs and medications may rise to toxic concentrations [9, 24, 87]. Common drugs which may be impacted by kavalactones include diazepam, caffeine, amitryptyline, imipramine, propranolol, fluoxetine, haloperidol, morphine and beta-blockers [14, 86]. A final interaction effect worth noting is that kava may compromise treatment for Parkinsons disease because it is a dopiminergic antagonist [11, 34]. To keep these potential risks in perspective no research to date has demonstrated the prevalence of drug interaction effects, but rather has identified that they hold potential for harm.
Risks associated with kava use practices
With all unregulated drugs there are risks related to: production (including storage); sale; and consumption of the drug. In regards to risks related to the production of kava, there is evidence of poor quality plant material in kava powder [3, 32, 56, 88] and concerns that pesticides may remain in harvested kava. The US Food and Drug Administration’s pesticide monitoring program analysed seven samples of kava in 2008 and found 57% violated the safe pesticide residue limits [89]. There is no monitoring of kava in Australia for pesticide or other contaminants, yet considering that kava is a largely unregulated crop there is potential for risks associated with contaminated kava to emerge. The effects of storage on kava are variable; kavalactones degrade over time thereby reducing potency [11]. This is unlikely to present a risk to kava users, however toxic moulds may be present in poorly stored kava and could therefore constitute a health risk [88].
In any unregulated market adulterants may be added to drugs to add weight and therefore increase profit margin. There is some anecdotal evidence of this in grey literature related to kava sold on the black market in Australia [21, 90] which presents a potential health risk. Finally there are health effects related to the way in which kava is prepared and consumed. Concerns that kava is often prepared and consumed in an unhygienic manner are well documented [19, 40, 91], as well as concerns that the prolonged kava drinking sessions may result in harms from long periods of sitting, dehydration, and not eating. These risks have not been systematically studied.
Conclusion
The research to date, including a systematic review into recreational kava use [45], demonstrates that kava may cause kava dermopathy, raised liver enzymes, nausea and weight loss in a dose dependent fashion. In addition kava has been associated with a range of other negative effects such as poor health, red eyes, low motivation, seizures and heart disease. There are also concerns related to the potential for kava dependence, risks associated with using kava, the potential for drug interaction effects and the impact of kava intoxication on motor coordination and the capacity to drive. To put these harms into context, Australian and international reviews have concluded that moderate levels of kava consumption present a low risk for harm. This raises the question of how to identify what ‘high levels’ of kava use are. Clough [92] proposed that 400 gm of kava per week was the level at which harms (negative effects) were most likely to occur. While this remains untested it is the best estimation to date [34]. Overall, there is insufficient evidence regarding the health effects of high levels of kava use; in particular prolonged heavy use. A need for continued research with controlled and well-designed experiments has been a recommendation from a range of inquiries and reports [9, 11, 21, 34, 36, 45, 80, 93].
Understanding kava use in Australia
Prevalence of kava use in Australia
The National Drug Strategy Household Survey (NDSHS) has collected data on kava since 1998, reporting consistently from 2001 that approximately 2% of the Australian population has used kava in the previous 12 months [94, 95]. Unfortunately in more recent surveys (2013 and 2016), data regarding kava is combined with ‘other’ psychoactive substances including inhalants and synthetic cannabis and specific findings have not been published [96, 97]. While there are limitations to what conclusions can be drawn from the data collected by the NDSHS due to small sample sizes, the results confirm that from a national perspective kava is consumed by a very small section of the Australian community.
Kava use among Aboriginal Australians
Kava is not a drug of concern for the vast majority of Aboriginal and Torres Strait Islander people in Australia. The National Aboriginal and Torres Strait Islander Social Survey (NATSISS) [98-100] provides valuable but limited data in relation to drug use; the NATSISS has a significant degree of under-coverage of the population, and there are particular concerns around the validity of the drug use questions due to methodological limitations [98-101]. Despite these concerns it remains an important source of data. Only 1.2% of respondents to the 2008 NATSISS reported past 12 month use of kava, with more males (1.7%) than females (0.7%) reporting kava use [102]. In contrast to this low prevalence at the national level kava has been consumed by Aboriginal people in Arnhem Land since the early 1980s [93] and concerns around high prevalence of use and associated harms have been discussed and been the topic of research from this time point. As a result, this review focuses on the prevalence and associated harms of kava only in Arnhem Land communities.
Kava use in Arnhem Land
Arnhem Land is located in the north east of the NT and includes the local government areas of West Arnhem and East Arnhem Land. The population of the region is approximately 16,000; 12,000 of these people are Yolgnu (Aboriginal) [103]. The town of Nhulunbuy is the region’s largest population centre; it was set up in the early 1970s as a mining town. Other major population centres include Yirrkala, Gunbalanya, Ramingining, and Maningrida [103]. A significant proportion of the Yolgnu population live in small homeland (or outstation) communities. The movement back to homeland communities occured in the early 1980s, many groups moved to very small settlements on their traditional lands, often to escape the problems (alcohol, petrol-sniffing, idleness) in the larger townships [103]. The size of homelands communities ranges from approximately 20 to 100 people [103].
Kava use occurs in communities in both East and West Arnhem Land, yet not in all communities. Eight major communities have a history of kava use; Yirrkala, Ramingining, Milingimbi, Galiwinku, Gapuwiyak, Minjilang, Warruwi, and Maningrida. It is also used in several homelands communities near Ramingining and in the Laynhapuy Homelands and has been noted at times on Groote Eylandt [21, 80]. Kava use has been observed at various times in other communities however this is typically related to small groups with links to the main kava-using communities.
Broadly speaking the use of kava, like other drugs and alcohol, is socially determined, and must be considered in this context. Aboriginal people experience greater disadvantage compared with non-Indigenous Australians on all social indicators. As summarised by Gray and Wilkes [104] this is a consequence of the historical and continuing impact of colonialism and dispossession which has resulted in many Aboriginal communities being impoverished, marginalised, discriminated against, and with inequitable access to necessary public and private services, particularly education, health and employment [12]. Higher levels of harmful drug use are a consequence of this trauma and exacerbate poor health status and social disruption [105].
It is important to understand the context within which kava was introduced and continues to be used. Kava was introduced to Arnhem Land at Yirrkala in 1982, following a cultural exchange between the Yolgnu people and a Fijian community [19]. It was thought that kava may provide an alternative to alcohol, reduce alcohol related harm and facilitate connection to the Church. The introduction of kava in Arnhem Land coincided with a period of rapid social change that is well documented elsewhere [19, 106]. Elements of change that are noteworthy include the commencement of bauxite mining resulting in a dramatic population increase of approximately 3,300 non-Indigenous people residing in the township of Nhulunbuy. During this time, the influx of the workers also led to the introduction of alcohol despite the requests from Yolgnu Elders to ban it from the area [107]. This period also saw the emergence of the Homelands Movement in which many Aboriginal people left the mission towns such as Yirrkala and returned to their ancestral lands.
Kava use quickly became common in Yirrkala and Warruwi and by 1984 had spread to Minjalang, Groote Eylandt, and later Gapuwiyak, Galliwinku, Millingimbi, Ramingining and the Laynhapuy and Ramingining homelands. At the point of introduction both Aboriginal and non-Aboriginal people encouraged the use of kava as an alternative to alcohol [35, 79, 108] to provide fellowship without the harms. Alexander et al. [19] commented that the support of kava use by churches, and tacit government approval undoubtedly had an impact on prevalence and patterns of kava use; set up favourable attitudes to kava and low expectations of harms. It also set the social context as people were encouraged to use kava during meetings and ceremonially, as it was regarded as a safe and socially acceptable substance. This context in which people were encouraged to drink kava instead of alcohol, rather than support communities to address the underlying determinants of alcohol-related harm is important to keep in mind.
In Arnhem Land, similar to the Pacific, kava is consumed in group settings with participants sitting in a circle and sharing kava from a communal central bowl [31]. Groups include both males and females [35]. Clough et al. [31] classified the social settings of kava use using participant observation research in one community. They identified that kava was used in social settings (card games, friends, household groups, people with regular income), settings with another purpose (ceremonies and celebrations and Elders circles), and occasionally people drinking kava alone. It was thought that the rapid adoption of kava use was in part related to the fellowship of drinking around the communal bowl [40].
The extent of kava use in Arnhem Land
In reviewing kava research conducted in Arnhem Land it is important to acknowledge the context in which it was undertaken. The majority of research was conducted between 1989 and 2002, during which time there were frequently changing regulatory frameworks surrounding kava. In Arnhem Land between 1982 and 2007 there were six different periods of regulation (which are discussed in more detail below):
- unregulated (1982-1990)
- licenced (1990-1993)
- unregulated (regulatory hiatus) (1994-1998)
- illegal (1998-2001)
- licensed – National Code of Kava Management (2002-2007)
- import restriction (2007- current).
The social and geographical context of Arnhem Land also places limitations on how research is conducted and interpreted:
- inter-community and intra-community population variability limits the generalisability of findings
- the small population sizes of many communities mean most studies are conducted with small sample sizes, which limit their generalisability and the capacity for detailed statistical analysis
- the frequently changing regulation of kava and alcohol and other government interventions in Arnhem Land communities confounds conclusions that can be drawn from research and may influence participants comfort in answering questions about use [109].
Keeping this in mind a number of studies were completed between 1989 and 2002 which provide a considerable repository of data in a small population and provide a useful description of the prevalence and changes in kava consumption in the region during that time period. This research has informed policy and debate for the last 30 years, and has primarily been conducted by Alan Clough – then from Menzies School of Health Research – and colleagues. Clough and colleagues have produced up to 15 papers including research papers, reviews, commentary papers and letters drawn from five different data sources. Research by Mathews et al. [40], Alexander et al. [19] and reports by d’Abbs [21, 80, 93] have also been instrumental in describing the effects of kava in Arnhem Land. There has been little publically reported research related to kava consumption since the early 2000s and none since the end of kava licensing in 2007. Consequently all the data regarding prevalence and patterns of use – including data collected incidentally in a cannabis research project [106, 110] – was collected prior to the commencement of kava licensing under National Code of Kava Management in 2002, with the exception of a letter published in the Medical Journal of Australia in 2006 [72].
Table 2 presents a summary of kava use conducted in Arnhem Land since 1986. The data in Table 2 demonstrate that since the late 1980s until 2002 there was a steady increase in the number of kava drinkers and in the average amounts consumed by drinkers in single sessions and over a week. It is evident from these data that kava use was more common among males than females, but that prevalence among females increased over the time period [80]. In communities in which kava use is present the prevalence among males was typically reported at around half or more than half of population (see Table 2). The results also showed that those using kava were likely to do so at harmful levels (>400g per week) [40, 69, 80].
Quantities of kava consumed in individual drinking sessions also changed over time. Alexander et al. [19] and Fleming, Watson, McDonald, and Alexander [111] reported that the average drinker would consume 1.6 litres of kava per sitting. By comparison Clough et al. [31] reported consumption at approximately seven cups per hour (0.7 L), over periods greater than 3 hours (resulting in at least 2.1 L) – suggesting an increase in volume consumed. The duration of kava consumption has been reported in a number of studies, with one study reporting a group session which lasted for approximately 14.4 hours [78].
Little is known about the course of kava use over an extended time; no longitudinal studies have been conducted. However, two studies [112, 113] looked specifically at younger adults and determined that kava prevalence was somewhat lower than in the broader adult community, suggesting that kava use was more prevalent among adults over the age of 30 years.
The lack of research data collected since 2002 make further observations difficult, therefore it is important to consider other indicators of kava use and availability. In 2006 Clough and colleagues [72] examined population survey data, police seizures of kava (calculated to account for 14% of illegal kava supplied) and wholesale data sales figures across different regulatory periods until 2006. The results demonstrated that a demand for kava persisted during all periods of regulation studied; that kava was at its lowest availability during 2000 and 2001 (when kava was illegal) and its highest availability when kava was unregulated and during licensing periods (including the period between 2002 and 2006 for which there is little data published) [72]. Despite a lack of detailed methodology related to the data it provides a useful guide to understanding the broad availability of kava.
Since Clough and colleagues’ 2006 commentary there has been no published data related to kava use in Arnhem Land. To gauge more current kava availability, evidence of black market activity is informative. NT police media and other media outlets report that kava seizures are continuing to be made and have been reported from 2007 until the current time [39, 114-118]. The seizures described in media reports include those large quantities (45 kg) that have been divided into deal bags – suggesting that the demand for kava remains and that the black market for kava is organised and consistently active. Despite this persistent demand, anecdotal evidence from a health worker focus group suggests that kava availability and consumption has decreased since the import restriction in 2007 [119]. However, concerns remain and anecdotal evidence of harmful use persists presented in media reports [115] [58]. The prevalence of kava use since 2009 is unknown, contributing to a lack of understanding of current quantities and frequencies of kava used – and whether there is any uptake of kava use among those who previously did not use. There is a need for more current data on the prevalence of kava use, and indeed other drug and alcohol use in Arnhem Land.
It is important to note that kava is not used in isolation. Despite differing regulations pertaining to alcohol and kava between communities, varying degrees of black markets for each drug exist, thus both drugs are available. In 2002 Clough et al. [109] collected data about both alcohol and kava. Their findings suggest that it was not uncommon for people to use both despite widespread belief that people choose kava or alcohol. There is no detail as to how the two are used together; whether at the same time or at different times. Kava use has also been documented among people using petrol and cannabis: Burns et al. [112] found that 38% of a sample of young people who sniffed petrol also reported kava use; Lee, Clough and Conigrave [120] report that 15% of cannabis users also reported kava use and that 7% of cannabis users would look for kava if cannabis was unavailable. Clough et al. [109] notes that a dynamic drug-use complex exists in Arnhem Land involving changing patterns of different drugs, including kava, alcohol, petrol and cannabis. These data underscore the need for intervention efforts to address the determinants of all drug use not simply individual drugs.
Table 2. Summary of findings of kava use research in Arnhem Land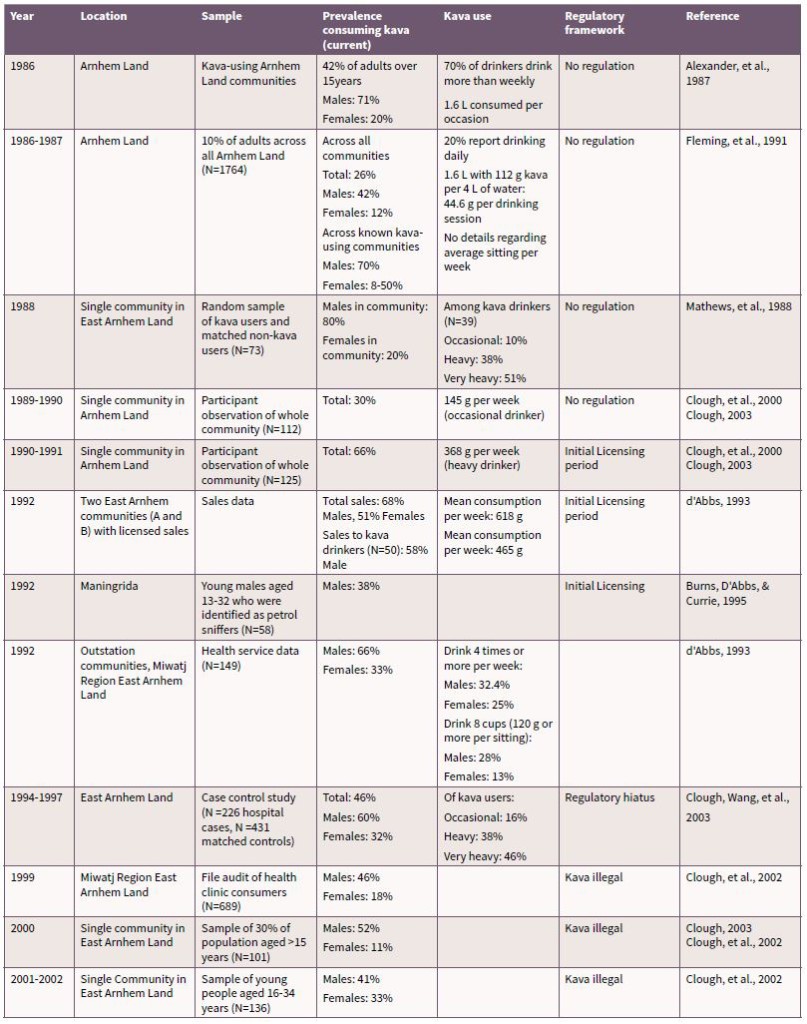
Health impacts of kava use in Arnhem Land communities
Impacts of kava use experienced by Arnhem Land communities
The documented consumption of kava in Arnhem Land (see Table 2) between 1989 and 2002 includes high proportions of people drinking more than 400g of kava per week, the level at which kava related harms are purported to emerge [92]. Indeed all the health effects identified by the systematic review [45] as caused or associated with kava use have been identified at some point in Arnhem Land communities with the exception of impotence. Impotence has not been addressed in any research to date or identified in commentary, so no conclusions can be made. There is no evidence that kava has had a beneficial impact on the health of Aboriginal people in Arnhem Land.
Due to the lack of recent research the following section reviews previous research and provides a summary of recent anecdotal reports to describe the effects of kava use on the health of kava-using Arnhem Land communities. The harms that have been identified across studies as the most prevalent are kava dermopathy, self-reported and clinician rated overall poor general health, low body weight, redness of the eyes, lethargy and ‘amotivation’, and raised liver enzymes [19, 26, 31, 40-42, 53, 65-69, 79, 80, 91, 92]. Given the kava use data available (see Table 2) these harms were likely experienced by a large proportion of kava users – which in turn is a large proportion of individual communities [92]. Indeed descriptive studies investigating the health effects of kava by Mathews et al. [40] and Clough et al. [42] show significant proportions of kava drinkers experience these negative effects. For example, kava dermopathy was identified in 45% of kava users by Clough et al. [42] and 60% of heavy users, and 70% of very heavy users in Mathews and colleagues’ study [40]. Similarly Clough et al. [42] found elevated liver enzymes in 61% and low BMI in 32% of kava using participants. Of Mathews et al. [40] participants who were either heavy or very heavy kava drinkers 36% and 39% respectively reported poor general health.
In addition to these established consequences there is some evidence from Arnhem Land concerning the effect of kava on immune functioning (increased susceptibility to diseases) [53, 68, 69], cardiovascular functioning [40, 53, 66], and the risk of seizures [65]. Additional health concerns linked to poor hygiene practices (unclean mixing utensils and bowls, and sharing drinking vessels) have also been identified in the research. It has been argued that this may increase the likelihood of disease transmission [80], but there is little substantiated evidence for these concerns.
It is not possible to ascertain current health impacts of kava use experienced in Arnhem communities. Anecdotal information collected through focus groups in Arnhem Land communities indicate that there has been a perceived reduction in presentations to health services consistent with heavy kava use (for example as kava dermopathy) [119]. That said kava remains available, there is still a demand for it and there are reports in the news media of groups who still use heavily and experience associated health harms such as low body weight, ‘amotivation’, and poor general health [115].
Impacts of kava on social and emotional wellbeing
The kava-inducing effects of relaxation and reduced anxiety were key drivers in its introduction and indeed were initially regarded as positive effects of kava on social and emotional wellbeing [108]. However, research has demonstrated that heavy kava use and the length of time spent consuming kava has had negative impacts on wellbeing [21, 93]. Clough and d’Abbs demonstrated that heavy kava use negatively effects the social and emotional wellbeing of individuals and families [21, 31, 91, 92]. Informants in d’Abbs’ and Burns’ 1993 [21] review observed that as kava use increased in communities, family disruption (including the neglect of, and reduced supervision of children, lack of preparation of food, neglect of family hygiene, and neglect of family duties) increased and individual participation in employment, community and cultural activities decreased. Potential mechanisms for role neglect include ‘amotivation’ and lethargy arising from kava consumption [15, 23] and that roles are neglected in favour of kava use – which is consistent with drug dependence criteria [73].
Heavy kava use has considerable economic impacts on individuals and families [21, 92, 121] when family finances are spent on kava. The extent of this impact is related to the regulatory framework in which kava is purchased – under the most recent licensing period kava retailed at $150 per kilo – a price which is lower and more stable than black market kava. Current estimates are that kava sells for around $1000 per kilo [116, 118], thus exacerbating economic effects for those who continue to use heavily. In addition to increased price, regulatory frameworks under which kava selling was unregulated or illegal (black market reliant) resulted in additional negative social effects. These included: bringing outside influences into communities; the high cost of kava; the potential for sub-standard kava, legal consequences for users; and consequences of black market activity (e.g. car accidents when driving at night to traffic kava) [21, 93].
The current prevalence of social harms are not known. Anecdotal evidence suggests that there are still heavy kava users in some communities who are experiencing social harms such as neglect of family roles and reduced participation in employment but this appears to have diminished [115, 119]. As no research to date has investigated the relationship between kava use and mood states or mental health in Arnhem Land communities, no conclusions can be drawn regarding the effect of kava use on mental health.
Impacts of kava on community wellbeing
The impacts of kava on community wellbeing has been a politically charged topic since its introduction – there has been much speculation as to its effects, both positive and negative. Social cohesion is proposed as a benefit of kava use – it is argued that the relaxing effects of kava coupled with a negligible impact on cognitive capacity creates a pleasant social environment and fellowship [5, 38, 108]. While this has not been tested empirically it has face validity. However, outside of the kava drinking circle there is little evidence of social cohesion arising from kava. As summarised in Table 2 there has been a high prevalence of heavy kava use in kava-using communities use in Arnhem Land which has resulted in large amounts of time spent consuming kava and large amounts of money being spent on kava. This has had negative impacts on community wellbeing and functioning [115, 122, 123].
The neglect of roles (family roles, traditional activities, cultural obligations) associated with kava use by a large proportion of a community – due to kava use – has had negative effects on community wellbeing. Research has shown a decline in community activity, cultural activity, community cleanliness, and participation in employment related to an increase in the prevalence of kava use [21, 31, 72, 79]. Indeed heavy kava use has been implicated in the collapse of a football competition [21], and low uptake of employment opportunities and low employee retention in local government programs [124].
Community level economic effects of heavy kava use have been documented [21, 92]. Clough [92] argued that the high prevalences of users in small communities created a cash drain. This has been particularly significant while kava use has been unregulated and illegal as the cost of kava has been higher and the money leaves the community. In contrast under licensing frameworks money spent on kava was retained in the community and was used to pursue community priorities [21] (see discussion under regulations section below). Secondary economic effects from lack of participation in employment and an increased need for health services have also been attributed to kava [91, 124].
Clough (2003) attempted to quantify the impacts of kava use on communities. He proposed that if more than half the males, and 20–40% of females are using kava, and if 20% of available cash goes to purchasing it, effects on community functioning are likely [92]. This summation has not been tested empirically so cannot be interpreted as a firm indicator of when harm will emerge, but it recognises the sheer toll on communities when a significant proportion of the population engages in heavy kava use and experiences harms such as overall poor health, lack of motivation, low body weight and reduced time spent in important roles.
In considering the effects of kava on community wellbeing a discussion of the relationship between kava and alcohol related harm is important. Kava was initially proposed to be beneficial in terms of what it does not cause in comparison to alcohol – such as violence. It is a common belief in Arnhem Land that kava reduces alcohol-related problems [19]. It is argued that the consumption of kava results in relaxation whereas alcohol intoxication can result in agitation and aggression, therefore by making kava available alcohol related harm will be diminished. The contrasting possibility that if kava is not available then alcohol related violence will increase is also cited in opinion pieces in popular media [122, 125, 126]. Furthermore community members have raised concerns that the availability of kava prevents men from leaving communities to seek alcohol.
There have been no systematic or well-designed descriptive studies examining the relationship between kava availability and alcohol related harm. Furthermore descriptive studies in Arnhem communities have typically been conducted in communities in which access to alcohol is limited [19, 40]. In these studies alcohol related harm is described as reduced, yet it is not clear if this is related to the presence of kava or alcohol restrictions. In an unpublished study, Chalmers [91] compared alcohol related arrests in two Arnhem communities, one that used kava and one that did not. The kava-using community did not have fewer alcohol related arrests compared to the non kava-using community. She concluded that alcohol related harms occur regardless of the prevalence of kava in communities [91]. It is important to note that although, the physical harms caused by kava are less than those caused by alcohol [127] the reality is that there is rarely a situation where only one is available, which makes the use of kava as an agent to minimise alcohol related harm unlikely to be effective over the long term. However it is important to acknowledge these concerns as they are areas of great public interest to affected communities. Finally, there has been extensive and ongoing debate about kava which itself has impacted community functioning; kava is a divisive community issue. McLeod [128] noted that the ongoing issues around kava, kava management and licensing have dominated local politics and community meeting agendas in some communities for years and this occurred to the neglect of other issues.
Regulation of kava in Australia
History of regulation in Australia and its impacts
Kava presents a considerable policy challenge to Australian authorities [129] and has a complex regulatory history. It has cultural significance for Pacific Islander Australians, it’s therapeutic use has growing evidence as an alternative medicine for anxiety and sleep disorders, it is an important crop for Australia’s Pacific neighbours and there is evidence that its overuse has negative impacts on Arnhem Land communities. As a consequence of these diverse and conflicting factors, kava has been reviewed by a range of national and state level authorities including: the Therapeutic Goods Administration, FSANZ and Consumer Protection. It has been listed in Australia as a food substance, a botanical, a Schedule 4 drug, and a poison at various points in time. Considering that the focus of this review is focussed on Arnhem Land communities the following discussion will focus primarily on regulation in the NT.
The regulation of kava has implications for patterns of kava use and its related harms, black market activity and its related harms, and demands on organisations which interact with kava use and its harms (e.g. health, licensing and policing). Furthermore the implementation of kava regulations themselves are a potential area for both harm and self-determination and community capacity building. Impacts of the different periods of kava regulation are integrated in to the summary below.
Regulations pre 1982-1990 – Unregulated
Prior to 1982, kava was classified as a food substance in the NT and there were no regulations on sale or supply. When use rapidly expanded, some communities adopted self-regulatory approaches including restricting sales, banning children from using kava and banning it completely [107]. At various stages between 1982 and 1991 kava was banned in five of the eight kava-using communities as part of local community decision making [80]. However communities had difficulty in exercising control over sales as demand for kava increased and non-Indigenous kava sellers entered the market [80]. During this period kava use increased both in terms of the number of people who used kava and the amount individuals were using. Not surprisingly kava-related harms emerged [19]. In contrast, as kava sellers attempted to expand kava sales into the Kimberley region of Western Australia (WA) around this time, local Elders raised concerns about the effects of kava and requested that the WA Government ban it [21]. The WA Government complied with this request and banned the sale and supply of kava by amending the Poisons Act [85]. The ban on the sale and supply of kava did not prohibit possession, thus allowing continued use by Pacific Islander peoples in WA. Arguably this rapid action, including condemnation by Elders and support through regulation, prevented kava use from taking hold [80]. Kava remained banned in WA under the Poisons Act until early 2017, allowing for the sale of alternative medicines containing kava.
Regulations 1990-1993
In May 1990, as a response to the emergence of both public concern and research documenting the negative health effects of kava (e.g. [40]), the Commonwealth and NT governments classified kava as a ‘dangerous good’. In addition the Australian Government’s Ministerial Council on Drug Strategy endorsed the recommendation that kava use should be actively discouraged [130], yet there is little record of what steps were taken at this point to discourage kava use outside the NT and WA . The classification enabled the NT to undertake its own regulations. A kava licensing system was established under the NT Consumer and Fair Trading Act. The goal of the legislation was to reduce the per capita consumption of kava and minimise kava-related harm [80]. Under the licensing system kava sales were permitted if the NT Minister for Health granted approval, at which point kava could only be sold by community-controlled organisations who were endorsed licensees. Licensees could only purchase kava from an agreed wholesaler. Five communities decided to sell licensed kava. Sale restrictions included age restrictions (> 18 years) and a daily maximum of 50 g [112]. Under this legislation the sale and supply of kava in the NT outside licensed areas was illegal but possession was not. Consequently it was particularly difficult for police and prosecutors to effectively prevent a black market from emerging [80].
This period of kava licensing was subject to an evaluation [80, 112]. In reviewing the initial licensing period d’Abbs [80] concluded that objectives of the control measures were appropriate, yet failed to meet their objective: they failed to reduce the per capita consumption of kava use; there was a lack of responsible sale of kava; and there was a lack of resources and regulations to prevent black marketing of kava. d’Abbs recommended changes to the licensing system including: more accurate retail systems; more responsible service and sale of kava; funds allocated to health promotion and research; and adequate resourcing for overseeing licence compliance [80].
1993-1998 – Regulatory hiatus
Under the licensing system kava use continued to grow and concerns regarding health effects continued to mount [80]. In response, the Commonwealth Government gazetted kava as a prohibited botanical under the Food Standards Act in 1994. This meant that the licensing laws in the NT contravened the Food Standards Act and as a consequence licensed retailers were no longer permitted to sell kava [107]. Thus the licensed trade ceased, the sale of kava became illegal in the NT and changes proposed by the evaluation were not enacted [107].
The Commonwealth did not undertake to enforce the new prohibition on importing kava, meaning that non-authorised suppliers were somewhat immune from any danger of prosecution [93]. Clough and Jones [107] describe this period as a ‘regulatory hiatus’ and it arguably created an opportunity for black marketers to emerge, and become entrenched in Arnhem Land communities [92, 107]. This period of unregulated availability undermined the participation of communities and the NT government in establishing kava licensing, and their capacity to minimise the harms associated with kava and respond to community concerns in a timely fashion [107]. In 1995 the NT government requested an exemption from the Commonwealth decision in order to maintain the licensing system in response to ongoing harms related to kava use. In response the National Food Authority (NFA) conducted an inquiry into kava.
The NFA’s inquiry took 12 months and culminated in the release of a Draft National Kava Management Strategy. The strategy comprised four components: a national system for restricting and monitoring the importation of kava; the National Code of Kava Management (NCKM) by which all importers, wholesalers, retailers and distributors must abide; a new addition to the Food Standards Code, which would apply only to kava; and an option for states and territories to impose their own, more restrictive legislation [11, 131]. From 1998 to 2001 the legislation to enable the NCKM was established. The goal of the legislation was to regulate, not prohibit, use and to ensure provision for cultural use [11].
Evidence suggests that during this period kava availability, and per capita consumption, was reduced, however demand remained and there was a steady increase in black market activity [72]. There is no clear evidence regarding how the banning of kava in the NT has impacted on other indices of kava-related harm, indeed prohibition hinders the collection of data to monitor kava usage. It is clear though, that kava bans dramatically increase the price of kava [121], which potentially results in large portions of family income being spent on kava and considerable amounts of money leaving the communities. Prohibition also meant that kava became a predominantly law enforcement responsibility, with Police receiving no extra resourcing to address it [121]. Furthermore, prohibition was not accompanied by significant funding or resourcing for health promotion or health interventions to support people who used kava prior to the ban.
2002-2007 – Second kava licensing period
Between the NCKM being developed and coming into effect, the NT government passed the Kava Management Act (KMA), which was founded on harm minimisation and prohibited the sale and consumption of kava except in licensed premises [132]. The licensing system built on the findings of the evaluation of the initial period of licensing [80]. The licensed sale of kava re-commenced under the KMA in 2002. It was hoped that the KMA could achieve the following objectives: the responsible sale of kava; retaining money spent on kava in communities; reduction in the black market; funding of health promotion, intervention and research; and monitoring of kava availability. Initially under the KMA three communities successfully applied for retail licences with another in 2004 and another in 2006. Importantly not all communities with a history of kava use chose to apply for a kava licence [132]. The Act stipulated that retail licensees could only purchase kava from one designated wholesaler (a community controlled organisation) and that individuals could purchase up to 800 gms per week (an amount double the level at which harms are thought to occur [92]). The NT licensing commission managed the Act by adopting a requirement for licensees to have a kava management plan in place [130, 133]. Management plans encouraged community self-management, and allowed communities to devise regulations applicable to their particular circumstances [121].
Management plans varied based on community needs but the key headings to be addressed were: boundary of licensed area; areas where the possession of kava should be prohibited; times and place of purchase; purchase limits; community expectations or rules; and actions to monitor and modify kava’s negative impacts [21, 121, 132]. Community consensus was required and the views of the Departments of Health, Communities and Police were also formally considered as part of the licensing process. Two of the five communities proposed to allow sale from Monday to Saturday with trading restricted to afternoon or evening hours. Two proposed to trade on specific days during the working week and to avoid paydays. Three communities proposed to keep a register of drinkers [21].
There was no formal evaluation of this licensing period despite extensive preparation in the development phase. The positive consequences noted during this licensing period included: safer drinking practices were encouraged; prices were fixed; annual licensee conferences were undertaken; and a Kava Education and Health Advisory Group was established [121]. Despite these advantages evidence suggests that it did not result in the desired reduction in kava-related harm and availability of kava continued to grow [72, 121]. It was an improvement on the first attempt at licensing, however unsafe levels of kava were still being sold, kava sales were steadily increasing, community harms were still prevalent and there was a lack of a coordinated health response [99].
Commentaries have identified a range of factors which enabled the continuation of kava-related harm [72, 92, 121] including the persistent illegal trade (in both kava licensed and unlicensed communities), evidence of underage drinking, communities becoming reliant on kava related income [134], and community concerns that kava was required to prevent alcohol related harm. Clearly the most concerning component of the licensing period was the weekly amount of kava allowed per person which was in contrast to the best evidence to date regarding safe levels of use [92]. Despite these problems, only controlled supply strategies have had ongoing community consultation, clear objectives, goals and mechanisms to monitor those objectives and goals. The importance of this collaborative approach cannot be understated. Both periods of licensing were terminated prior to those objectives and goals being adjusted in light of data suggesting increased sales and harms, which has been challenging for communities working to manage kava related harms and to moderate use.
2007 and beyond – Importation restriction
In 2007, the Australian Government imposed restrictions on kava importation [135]. As a result, all legal sales in the NT ceased. Although there is currently a ban on the importation of kava, the Kava Management Act is still in force in the NT [135]; this includes the provision of punishments for possession and supply. Possession without a license carries a fine of up to $10,000 and two years imprisonment for between 2–25 kg. Amounts less than 2 kg can be confiscated and disposed of by the Police without prosecution. Kava supply (quantities greater than 25 kg) carries a penalty of up to eight years imprisonment or 14 years if supplied to a minor [135].
Currently kava remains on the prohibited and restricted imports list under the Customs (Prohibited Imports) Regulations 1956 Act [136]. Under the Act, an exemption allows a passenger to import up to 2 kg of kava in either root or dried powder form in their accompanied baggage without a permit, if they are on a ship or aircraft and aged 18 years or more. This exemption does not apply to kava being imported via post, courier services or unaccompanied baggage [136]. Finally it is currently listed in Australia as a Schedule 4 drug by the Therapeutic Goods Administration (TGA) in relation to the use of kava extract in therapeutic (natural) medicine [137-139].
The current kava import restriction is largely consistent with previous kava bans in Arnhem Land communities, and relies on law enforcement as the primary strategy to minimise kava-related harm. The lack of consultation prior to the current import restriction and its rapid implementation has had a negative effect on communities, both financially and because it undermined community self-determination [134]. There has been no publically released research into the effects of the importation ban; grey literature provides the primary source of information [140, 141]. To complicate matters the kava import restriction coincided with the controversial Northern Territory Emergency Response (NTER) implemented by the Australian Government [142] and a range of changes commenced by the NT Government, including the amalgamation of small community councils into larger regional shires. These policy changes create a confound in understanding kava availability, kava related harm, and the context in which kava is now used. The NTER was a top down, largely non-consultative policy approach [142-144]. Similarly the import restriction, although foreshadowed publically [72, 145], was implemented with little forewarning to affected communities. Prior to the import restriction no formal review process or consultation was conducted. For some communities the first they knew of the ban was the day after it came into place [90, 146]. The lack of consultation during this period undermined many of the efforts that were being undertaken as part of the KMA.
Documented initial consequences of the importation ban on communities are largely related to the financial impacts of the sudden end of the trade [134]. It resulted in a significant loss of discretionary income used for community benefit [143]. Kava profits assisted with the expense of managing remote communities in the absence of ongoing funding [141]. Retail licensees and the wholesaler were angered that agencies received no compensation for loss of income [90]. This lack of compensation made it difficult for agencies to continue operations – kava income had become a crucial part of service delivery. There has been no publically released research into the health and social effects of the importation ban over either the short or the long term.
Concerns that the kava ban would bring about a return to alcohol consumption were expressed widely [143] yet no research has been undertaken. An important criticism levelled at the ban was that it occurred prior to the implementation of effective alcohol management [146]. Hughes [121] cautioned that the ending of kava licensing in Arnhem Land must be accompanied by education, access to jobs, good health and decent housing, arguing that if these conditions were not addressed, the result may be greater consumption of alcohol, cannabis and other drugs. In support of these arguments a newspaper article published shortly following the ban [122] reported that alcohol-related crime and gambling had increased in areas which had previously had kava. In contrast, another article [125] argued that an increase in alcohol could be better accounted for by a local sports event and funeral than the kava ban. Consistent with these contrasting views, in a review of remote policing, Delahunty and Putt [147] cautioned that success in reducing community spending on any particular drug or gambling may leave more cash for food and other essentials but noted that the extra cash might also create new opportunities for illegal cannabis or alcohol trades.
There are two additional concerns associated with the rapid end to licensed kava. Firstly, the ban was not accompanied by additional policing resources to prevent the resurgence of the black market. Policing the black market is challenging not only due to the complexity of remote area policing [147] but also because kava trafficking is not prioritised by jurisdictions (outside of the NT) from which kava originates [115]. Secondly, there were no additional resources provided to health and drug and alcohol services to assist current users of kava. Considering concerns that a kava withdrawal syndrome may include seizures [65] a sudden ban with no resources and no support provided to health practitioners was a risky, short sighted and irresponsible move [47].
There is no published evidence regarding the outcomes of the ban. Anecdotal reports suggest that the ongoing effects of kava prohibition have been a reduction in overall kava availability and harms [119], but that heavy kava use is still present, albeit more hidden [115]. As a stand-alone measure to increase the health and wellbeing of Arnhem Land communities the importation restriction on kava likely had minimal effect. Any efforts to decrease drug use must be accompanied by efforts to address the drivers to the misuse of drugs; this includes the social determinants of health and addressing the broader disadvantage confronted by Aboriginal communities in Arnhem Land in the areas of education, health, employment, housing and self-determination [104, 121, 148]. Some community organisations have argued that the decision to ban kava took power away from the community [134, 146] and prevented changes being made to the licensing structure in response to evidence of ongoing harm. Regardless of whether the tightening of the importation restrictions resulted in a reduction in kava availability, the process by which the ban was implemented undermined Arnhem Land communities.
Future developments – 2019 and beyond
In January 2019 the current Prime Minister, Scott Morrison, proposed changing the importation ban on kava through the development of a pilot program [149, 150]. This policy change is in stark contrast to the Government’s previous position on kava [145, 150, 151] and has been made in the context of supporting the economies of Australia’s Pacific neighbours; in particular Vanuatu. Detail regarding the pilot program are yet to be developed, although a call for consultation has been released [152], which suggests an increase in the import restriction from 2 kg to 4 kg per person entering Australia. There are some reports that the pilot program will allow states and territories to uphold bans that are currently in place [151]. Community responses and Northern Territory Police responses to the potential changes in import restriction reported in the news media stress that Aboriginal communities in Arnhem Land have yet to be consulted on the change and there are concerns that it may lead to an increase in availability of kava and related harms [151].
Strategies to address kava related harm
Approaches to limit harmful kava use in Arnhem Land have been largely limited to supply reduction regulations as described above. Put simply, there are no intervention options, either nationally or internationally, described in the literature to reduce harmful consequences of kava use. The development, evaluation and dissemination of interventions to support people who experience kava related harms is lacking. In Arnhem Land demand and harm reduction interventions have been poorly funded and executed in the case of licensing periods, or completely lacking during periods when kava has been banned or unregulated. Existing interventions in Arnhem Land communities have been limited by a general lack of resourcing to, and planning of, drug and alcohol services and primary health care in general, and the challenges of remote area service delivery [153]. Several health promotion interventions exist and include those that provide information about the effects of kava, and those that provide harm minimisation information (including efforts to promote responsible consumption, good hygiene and prevent the use of kava by pregnant women). None of these resources have been evaluated, and most appear to be not currently available, either online or to order hard copies. The majority of interventions to date have been conducted by primary health care services and include regular screening for kava use, health checks for kava users which include liver function tests, and training for health staff about the effects of kava use [91]. Clinical protocols for kava were developed in 2004 [154, 155] and provide very brief screening advice for health practitioners. There are no specialised kava treatment services available in the NT and there is limited support beyond health promotion resources provided to existing services. There has been no training or resource development since the implementation of the import restriction meaning that health services are not necessarily supported to deal with fluctuations in kava related harms.
In the absence of an evidence base for kava use, general best practise recommendations for alcohol and drug interventions in Aboriginal communities should be applied to kava related harms (e.g. [104]). As summarised by Gray and colleagues [104] drug use is complex and multi-causal. Addressing it requires a comprehensive approach, including strategies to: address the underlying social determinants; prevent or minimise the uptake of harmful use; provide treatment for those who are dependent; and support those whose lives are affected by others’ harmful AOD use. Although supply reduction is clearly an important component to reduce the harmful effects of kava use, regulations to date have relied too heavily on supply reduction without addressing other determinants of use, and not surprisingly have had limited efficacy.
Future directions
This review has identified several possible avenues for future research and action:
- There is a need for ongoing high quality research into the effects of recreational kava use on health and wellbeing across different contexts, particularly the long term effects of high levels of kava use and the characteristics of kava dependence, tolerance and withdrawal. Without this research the development of evidence based policy, health promotion and treatment guidelines is made more difficult.
- There is a need for updated data on the prevalence and pattern of kava use across Arnhem Land communities. Importantly, considering the complex interplay with different drugs across communities such as alcohol and cannabis, as described by Clough [106], research should not focus solely on kava.
- Under the current regulations, efforts to minimise harms associated with black market kava are required. There needs to be an ongoing effort to prevent kava trafficking both within the NT and other jurisdictions from which kava originates.
- Any efforts to reduce the availability of kava needs to be coupled with the availability of support for current kava users. This is in conjunction with a broader need to address the social determinants of drug use at the community and individual level.
- The way in which some elements of kava policy have been implemented (particularly the 2007 import restriction) has caused harm. Collaborative community engagement is required to inform policy, change policy and also to implement it.
- Finally, there is a need to adequately resource an evaluation of kava regulations.
Concluding comments
This review has summarised information about kava, with particular attention paid to its potential to affect health. While a lack of research data has been identified, the best evidence to date suggests that at low and moderate levels of use kava presents a low risk for harm. However when used in higher amounts (>400 gm per week) it places those who use at risk of health problems. The harmful effects for which there is the most evidence are kava dermopathy, low body mass, and elevated liver enzymes. In addition kava use is associated with poor general health, lowered immune functioning, the potential for cardiac problems and risk of seizures.
This review examined kava use in Arnhem Land, its history, prevalence and effects on the health and wellbeing of individuals and communities. It highlights a lack of research, particularly recent research, however the evidence presented demonstrates that kava has been used at levels consistent with harm by a significant proportion of community members within kava-using communities between 1982 and 2002. During this period kava-related health effects and social effects were noted at the community and individual level. Social effects of note include reduce participation in family, community and cultural life and cash drains on families and whole of communities. There is a lack of research from 2007 onwards from which to examine the extent of kava use and related harms. Anecdotal evidence suggests kava is still being used and harms experienced, although not to the level that was seen between 1982 and 2002. Black market activity is ongoing and presents its own risks. Furthermore, kava use occurs in a complex dynamic of social issues and polydrug use that may exacerbate its impacts but also make it hard to separate the specific role that kava plays in health and wellbeing.
A variety of regulations have been implemented in an attempt to minimise the harms caused by kava use. The review has provided an overview of kava regulation in Australia and its current regulatory status. Kava has a complex regulatory history with only one regulatory period being subjected to evaluation. The two periods of licensing implemented in the NT have not sufficiently minimised harm – however authorities were not afforded the opportunity to adapt the regulations following evidence of increasing consumption. Current legislation prevents the sale of kava in Australia. This has reduced availability but there is an ongoing demand and a well organised black market. The review has also outlined the implications of how legislation has been implemented highlighting the need for policy to be developed in a collaborative way such that it does not itself cause harm. The review also notes that there is currently a possibility that the import restriction will be lifted or changed.
The review highlights the need for an increase in services available to people who use kava, to minimise harms associated with use, or to seek treatment. Additionally the review highlights the need for evidence based policy approaches (and implementation of policy) which considers the social determinants of drug use. These approaches must be undertaken in a way that ensures community ownership and self-determination, to address not only kava use but the overall health and wellbeing of Arnhem Land communities.
References
- Cairney, S. J., Maruff, P., & Clough, A. R. (2002). The neurobehavioural effects of kava. Australian and New Zealand Journal of Psychiatry, 36, 657-662.
- Shimoda, L. M. N., Showman, A. F., Baker, J. D., Lange, I., Koomoa, D. L., Stokes, A. J., & et al. (2015). Differential regulation of calcium signalling pathways by components of piper methysticum (‘awa). Phytotherapy Research, 29, 582-590.
- Lebot, V., & Legendre, L. (2016). Comparison of kava (Piper methysticum Forst.) varieties by UV absorbance of acetonic extracts and high-performance thin-layer chromatography. Journal of Food Composition and Analysis, 2016(48), 25-33.
- Rudgley, R. (1003). The alchemy of culture: intoxicants in society. London: British Museum Press.
- Singh, Y. N. (2004). An introduction to Kava Piper methysiticum. In Y. N. Singh (Ed.), From ethnology to pharmacology. Medicinal and aromatic plants – industrial profiles. Boca Raton: CRC Press.
- Singh, Y. N. (2004). Botany and ethnobotany of kava. In Y. N. Singh (Ed.), Kava: from ethnobotany to pharmacology. Medicinal and aromatic plants – industrial profiles. Boca Raton, USA: CRC Press.
- Lebot, V., Merlin, M., & Lindstrom, L. (1992). Kava: the Pacific drug. New Haven: Yale University Press.
- Pittler, M. H., & Ernst, E. (2003). Kava extract for treating anxiety. Cochrane Database of Systematic Reviews(CD003383).
- Sarris, J., LaPorte, E., & Schweitzer, I. (2011). Kava: a comprehensive review of efficacy, safety, and psychopharmacology. Australian and New Zealand Journal of Psychiatry, 45, 27-35.
- Food and Agriculture Organization of the United Nations (FAO). (2016). Kava: a review of the safety of traditional and recreational beverage consumption: technical report. Rome: World Health Organization.
- Food Standards Australia New Zealand. (2005). Kava: a human health risk assessment. Canberra: Food Standards Australia New Zealand.
- Mack, R. B. (1999). A less than Pacific Odyssey: the use of kava. North Carolina Medical Journal, 60, 91-93.
- Ramzan, I., & Tran, V. (2004). Chemistry of kava and kavalactones. In Y. N. Singh (Ed.), Kava: from ethnobotany to pharmacology. Medicinal and aromatic plants – industrial profiles. Boca Raton: CRC Press.
- Singh, Y. N. (2004). Pharmacology and Toxicology of kava and kavalactones. In Y. N. Singh (Ed.), Kava: from ethnobotany to pharmacology. Medicinal and aromatic plants – industrial profiles. Boca Raton.: CRC Press.
- Aporosa, S. G. (2008). Na yaqona kei na vuli: E rau veicoqacoca? Yaqona and education in Fiji: A clash of cultures. Domodomo: A Scholarly Journal of the Fijian Museum, 21, 7-30.
- Frater, A. S. (1976). Medical aspects of yaqona. Fiji Medical Journal, 111, 526-530.
- Foo, H., & Lemon, J. (1997). Acute effects of kava, alone or in combination with alcohol, on subjective measures of impairment and intoxication and on cognitive performance. Drug and Alcohol Review, 16, 147-155.
- Thompson, R., Ruch, W., & Hasenohrl, R. U. (2004). Enhanced cognitive performance and cheerful mood by standardized extracts of Piper methusticum (Kava kava). Human Psychopharmacology, 19, 243-250.
- Alexander, K., Watson, C., & Fleming, J. (1987). Kava in the North: A research report on current patterns of kava use in Arnhem Land Aboriginal Communities. Darwin: Australian National University, North Australia Unit.
- Beaglehole, E., & Beaglehole, P. (1941). Village in Tonga. Memoirs of the Polynesian Society. Wellington.
- d’Abbs, P., & Burns, C. B. (1997). Draft report on inquiry into the issue of kava regulation. Darwin: Menzies School of Health Research.
- Lemert, E. M. (1967). Secular use of kava in Tonga. Quarterly Journal of Studies on Alcohol, 28, 328-341.
- Tomlinson, M. (2006). A consuming tradition: kava drinking in Fiji. Expedition, 48(3), 8-17.
- Fu, P. P., Xia, Q., Guo, L., Yu, H., & Chan, P. (2008). Toxicity of Kava Kava. Journal of Environmental Science and Health, Part C, 26(1), 89-112.
- Ang-Lee, M. K., Moss, J., & Yuan, C. (2001). Herbal medicines and perioperative care. Journal of the American Medical Association, 286, 208-216.
- Bruner, N. R., & Anderson, K. G. (2009). Discriminative-stimulus and time-course effects of kava-kava (Piper methysticum) in rats. Pharmacology, Biochemistry and Behavior, 92, 297-303.
- Duffield, P. H., & Jamieson, D. (1991). Development of tolerance to kava in mice. Clinical and Experimental Pharmacology and Physiology, 18, 571-578.
- Jussofie, A., Schmiz, A., & Hiemke, C. (1994). Kavapyrone enriched extract from Piper methysticum as a modulator of the GABA binding sire in different regions of rat brain. Psychopharmacology, 116, 469-474.
- Davies, L. P., Drew, C. A., Duffield, P. H., Johnston, G. A. R., & Jamieson, D. (1992). Kava pyrones and resin: Studies on GABAA, GABAB and benzodiapine binding sites in rodent brain. Pharmacology and Toxicology, 71, 120-126.
- Showman, A. F., Baker, J. D., Linares, C., Naeole, C. K., Borris, R., Johnston, E., & et al. (2004). Contemporary Pacific and Western perspectives on ‘awa (Piper methysticum) toxicology. Fitoterapia, 100, 56-67.
- Clough, A. R., Burns, C. B., & Mununggurr, N. (2000). Kava in Arnhem Land: a review of consumption and its social correlates. Drug and Alcohol Review, 19(3), 319-328.
- Teschke, R., Sarris, J., & Lebot, V. (2011). Kava hepatotoxicity solution: a six point plan for new kava standardization. Phytomedicine, 18, 96-103.
- Nerurkur, P. V., Dragull, K., & Tans, C. (2004). In vitro toxicity of kava alkaloid, pipermethystine, in HepG2 cells compared to kavalactones.Toxicological Sciences, 79, 106-111.
- World Health Organization. (2007). Assessment of the risk of hepatotoxicity with kava products. Geneva: World Health Organization.
- Cawte, J. (1986). Parameters of Kava used as a challenge to alcohol. Australian and New Zealand Journal of Psychiatry, 20, 70-76.
- Jowitt, A., & Binihi, J. (2001). The commercialisation of kava in Vanuatu. Pacific Health Dialog, 8, 29-37.
- Kava R. (2001). The adverse effects of kava. Pacific Health Dialog, 8, 115-118.
- Grace, R. F. (2003). Kava drinking in Vanuatu – a hospital based survey. Pacific Health Dialog, 10(2), 41-43.
- Silaitoga, S. (2019). Cut down kava consumption; spend more time with family: Sigarara,
- Mathews, J., Riley, M., Fejo, L., Munoz, E., Milns, N., Gardner, I., . . . Gununuwawuy, B. (1988). Effects of the heavy usage of kava on physical health: summary of a pilot survey in an Aboriginal community. Medical Journal of Australia, 148, 548-555.
- Clough, A. R., Bailie, R. S., & Currie, B. (2003). Liver function test abnormalities in users of aqueous Kava extracts. Clinical Toxicology, 41(6), 821-829.
- Clough, A. R., Jacups, S. P., Wang, Z., Burns, C. B., Bailie, R., Cairney, S. J., . . . Currie, B. (2003). Health effects of kava use in eastern Arnhem Land Aboriginal community. Internal Medicine Journal, 33(8), 336-340.
- McDonald, D., & Jowitt, A. (2000). Kava in the Pacific Islands: a contemporary drug of abuse? Drug and Alcohol Review, 19, 217-227.
- Schmich, L., & Power, R. (2010). Situational analysis of drug and alcohol issues and responses in the pacific 2008-09. Canberra: Australian National Council on Drugs.
- Rychetnik, L., & Madronio, C. M. (2011). The health and social effects of drinking water-based infusions of kava: a review of the evidence. Drug and Alcohol Review, 30(1), 74-83.
- National Health and Medical Research Council. (2009). NHMRC levels of evidence and grades for recommendations for developers of guidelines. Canberra: National Health and Medical Research Council.
- Urquhart, B., & Thomson, N. (2009). Review of the misuse of kava among Indigenous people. Australian Indigenous HealthBulletin, 9(3), 1-14.
- Tomlinson, M. (2007). Everything and its opposite: kava drinking in Fiji. Anthropological Quarterly, 80, 1065-1080.
- Parker, S. (1994). Kava concern hits Fiji. Pacific Islands Monthly, 17.
- Gounder, R. (2006). Kava consumption and its health effects. Pacific Public Health, 13(2), 131-135.
- Heyes, C., Tait, C., Toholka, R., & Gebauer, K. (2014). Non-infectious skin disease in Indigenous Australians. Australasian Journal of Dermatology, 55(3), 176–184.
- Ruze, P. (1990). Kava-induced dermopathy: a niacin deficiency? The Lancet, 335(8703), 1442-1445.
- Clough, A. R., Rowley, K., & O’Dea, K. (2004). Kava use, dyslipidaemia and biomarkers of dietry quality in Aboriginal people in Arnhem Land in the Northern Territory (NT), Australia. European Journal of Clinical Nutrition, 58, 1090-1093.
- Norton, S. A., & Ruze, P. (1994). Kava dermopathy. Journal of the American Academy of Dermatology, 31(1), 89-97.
- Brown, A. C., Onop, J., Holck, P., Kufusi, P., Kabasawa, D., Craig, W. J., & et al. (2007). Traditional kava beverage consumption and liver function tests in a predominantly Tongan population in Hawaii. Clinical Toxicology, 45, 549-556.
- Russmann, S., Barguil, Y., Cabalion, P., Kritsandia, M., Duhet, D., & Lauterberg, B. H. (2003). Hepatic injury due to traditional aqueous extracts of the kava root in New Caledonia. European Journal of Gastroenterology and Hepatology, 15, 1033-1036.
- Teschke, R., & Wolff, A. (2009). Kava hepatotoxicity: Regulatory data selection and causality assessment. Digestive and Liver Disease, 41, 891-901.
- Ernst, E. (2004). Kava update: a European perspective. The New Zealand Medical Journal, 117(1205).
- Stickel, F., Baumüller, K., Seitz, K., Vasilakis, D., Seitz, G., Seitz, H. K., & Schuppan, D. (2003). Hepatitis induced by kava (piper methysticum rhizoma). Journal of Hepatology, 39(1), 62-67.
- Clough, A. R. (2005). Associations between tobacco and cannabis use in remote Indigenous populations in Northern Australia. Addiction, 100(3), 346-353.
- Ngirasowei, J., & Malani, J. (1998). The relationship between Sakau (kava) and gastritis. Pacific Health Dialog, 5(2), 266-267.
- Garner, L. F., & Klinger, J. D. (1985). Some visual effects caused by the beverage kava. Journal of Ethnopharmacology, 13, 307-311.
- Wheatley, D. (2001). Kava and Valerian in the treatment of stress-induced insomnia. Phytotherapy Research, 15(6), 549-551.
- Spillane PK, Fisher DA, & Currie BJ. (1997). Neurological manifestations of kava intoxication. Medical Journal of Australia, 167, 172-173.
- Clough, A., Cairney, S., Maruff, P., Burns, C., & Currie, B. (2001). Possible toxicity and withdrawal seizures in Aboriginal kava drinkers in Arnhem Land (Australia). South Pacific Journal of Psychology, 13(1), 27-34.
- Young, M. C., Fricker, P. F., Thomson, N. J., & Lee, K. A. P. (1999). Sudden death due to ischaemic heart disease in young Aboriginal sportsmen in the Northern Territory, 1982-1996. Medical Journal of Australia, 170(9), 425-428.
- Currie, B. (1997). Meliodosis: kava drinking added to risk factors? Northern Territory Communicable Diseases Bulletin, 3(4), 11-12.
- Currie, B. J., Fisher, D. A., Howard, D. M., Burrow, J. N. C., Lo, D., Selva-Nayagam, S., . . . Krause, V. L. (2000). Endemic melioidosis in tropical northern Australia: a 10-year prospective study and review of the literature. Clinical Infectious Diseases, 31, 981-986.
- Clough, A. R., Wang, Z., Bailie, R. S., Burns, C. B., & Currie, B. J. (2003). Case-control study of the association between kava use and pneumonia in eastern Arnhem and Aboriginal communities (Northern Territory, Australia). Epidemiology and Infection, 131(1), 627-635.
- Alexander, K., Watson, C., & Fleming, J. (1988). Kava in the north: a study of kava in Arnhem Land Aboriginal communities. Aboriginal Health Information Bulletin, 10, 32-37.
- Aporosa, S. G., & Tomlinson, M. (2014). Kava hangover and gold-standard science. Anthropologica, 56(1), 163-175.
- Clough, A. R., Currie, B. J., Yunupingu, M. W., & Conigrave, K. M. (2006). Action is required to reduce kava supply in Arnhem Land…again! [letter]. Medical Journal of Australia, 184(2), 91-92.
- World Health Organization. (1992). The ICD-10 classification of mental and behavioural disorders. Geneva: World Health Organization.
- Prescott, J., Jamieson, D., Emdur, N., & Duffield, P. H. (1993). Acute effects of kava on measures of cognitive performance, phsyiological function and mood. Drug and Alcohol Review, 12, 49-58.
1 Levels of evidence describe the quality of research that have investigated a topic: the highest level of evidence is Level I which denotes a systematic review of randomised control trials; Level II is evidence obtained from at least one randomised control trial; Level III – 1 is evidence obtained from a well designed pseudo-randomised controlled trial; Level III -2 is evidence obtained from a comparative study with concurrent controls; Level III-3 is evidence obtained from a comparative study without concurrent controls; Level IV is evidence obtained from a case series with either post-test or pre- post-test; and Level V is evidence obtained from non analytic studies (case report, expert opinion, consensus documents).