Review of kidney disease among Indigenous people (peer reviewed)
ReviewStumpers S1, Thomson N1
Review of kidney disease among Indigenous people. Australian Indigenous HealthBulletin 13(2). Retrieved [access date] from https://healthbulletin.org.au/articles/review-of-kidney-disease-among-indigenous-people
1Australian Indigenous HealthInfoNet
Sasha Stumpers, the principal author of this review, passed away unexpectedly on 23 September 2012. A well-liked and highly esteemed member of the HealthInfoNet team, Sasha is deeply missed.
View PDF version (PDF – 538 KB)
Introduction
Kidney disease is a significant health problem for all Australians, but severe kidney disease is more common among Indigenous people than among non-Indigenous people [1][2]. In particular, the prevalence of chronic kidney disease (CKD) (Box 1) [2][3][4][5][6] and the overall levels of end-stage kidney disease (ESKD) among Aboriginal and Torres Strait Islander peoples are consistently reported as significantly higher than among other Australians [4][7].
Box 1. Chronic kidney disease and end-stage kidney disease
Chronic kidney disease (CKD) is defined as kidney damage or reduced kidney function that lasts for three months or more [7][8][9]. Diabetes (diabetic nephropathy) and high blood pressure are the most common causes of CKD; other causes include glomerular disease, inherited disorders (such as polycystic kidney disease), and hypertensive renal disease.
The most severe form of chronic kidney disease, known as end-stage kidney disease (ESKD; also known as end-stage renal disease (ESRD)), occurs when kidney function has decreased to the point where kidney replacement therapy (KRT) is necessary to avoid death. KRT involves either dialysis (mechanical filtering of the blood to help maintain functions normally performed by the kidneys) or transplantation (implantation of a kidney from either a living or recently deceased donor). (See also Box 8.)
CKD is expensive to treat and has a marked impact on the quality of life of those who suffer from the disease as well as those who care for them [7][8]
Information on CKD among Indigenous Australians is available from self-reported survey data, as well as from community-based studies and screening programs [10][11][12][13][14][15][16][17][18][19][20] but the main focus in the literature has been on ESKD [4][7]. The incidence of ESKD is especially high for Indigenous people living in remote and very remote areas of Australia (Derived from [5]) with rates of ESKD highest in northern Australian Indigenous communities [4][6][21][22].
People with CKD require extensive hospital services, particularly those patients with ESKD who require kidney replacement therapy (KRT) to survive. As such, CKD is a significant cause of hospitalisation for the Indigenous Australian population [23]; this is particularly the case for dialysis, the form of KRT on which far greater proportions of Indigenous people with ESKD than their non-Indigenous counterparts rely [4]. In 2009-10, care involving dialysis was the most common reason for the hospitalisation of Indigenous Australians: they were hospitalised at 11 times the rate of other Australians [24].
Indigenous people have substantially higher death rates than other Australians from most causes [25][26] and diseases of the kidney and urinary system are one of the top ten leading causes of death overall, for all Australians, including Indigenous people [25]. Indigenous people are more likely to die from kidney disease that non-Indigenous people, with the death rate ratios being particularly high after the age of 25 years for both Indigenous males and females compared with rates for non-Indigenous Australians [7][27].
About this review
The purpose of this review is to provide an overview of the burden of kidney disease among Indigenous Australians. After summarising kidney disorders and the context of kidney disease among the general Australian population, attention is directed to the factors contributing to kidney disease among Indigenous people. The extent of CKD and ESKD among Indigenous people in Australia is then addressed by outlining the overall incidence and prevalence, and associated hospitalisation and mortality. The review also outlines the extent of other urologic conditions and disorders of the urinary tract among Indigenous people, including acute post-streptococcal glomerulonephritis (APSGN), urinary tract infections (UTIs) and urolithiasis. The factors contributing to kidney health problems, particularly CKD and ESKD, are considered in the context of the management and treatment of these conditions. The review concludes by outlining the current policies and strategies employed to address kidney health in Australia, with particular focus on polices and strategies employed to address the kidney health of Indigenous Australians.
Kidney disorders
‘Kidney disease’, ‘renal disease’ and ‘renal disorder’ are collective terms that refer to a variety of different renal and urologic disease processes involving damage to the working units of the kidneys and also includes conditions affecting the function of the body’s urinary system, involving the ureters, bladder and urethra [9]. The loss of kidney function (Box 2) can have a serious impact on the body, causing damage to other organs and bodily processes and, as a result, a number of complications and co-morbidities often occur, including: heart disease; infections; as well as problems with bones and muscles [7][28].
Box 2. The kidneys and their function
The two kidneys, parts of the urinary tract system, regulate the mineral composition, water content and acidity of the body [9]. They are also involved in the excretion of metabolic waste products and chemicals, are responsible for the production of certain hormones and vitamins, and also have a key role in blood pressure regulation. Removal of wastes occurs in tiny units inside the kidney known as nephrons; inside each nephron is a glomerulus which acts as a sieve-like filtering unit keeping proteins and cells in the bloodstream while allowing wastes to pass through. These wastes and any extra water become urine, which passes through tubes called the ureters into the bladder where it is stored until released during urination. Damage to the working units of the kidneys results in a reduction in the filtering capacity of one or both kidneys [9][29]. Progressive or repeated damage ultimately leads to a point where the kidneys are unable to maintain life, leading to death from ESKD unless a form of renal replacement therapy (dialysis or transplantation) is used.
Due to the commonality of the risks factors for other chronic diseases (such as diabetes, hypertension and other cardiovascular disease), kidney disease often occurs with concurrent or co-existing conditions [7][29][30]. Co-morbidities can appear or be worsened because of CKD, or they can arise independently. They contribute greatly to the development of CKD and ESKD [10][12][29]. Treatment required for those who have reached the stage of ESKD involves KRT (also known as renal replacement therapy (RRT)), involving transplantation of the kidney or dialysis [9][23].
Other important kidney and urologic disorders include APSGN, UTIs, and urolithiasis [31][32][33][34]. The unusual epidemiology of urolithiasis within Indigenous communities and the potential for recurrent or persistent UTIs to trigger more serious kidney damage when coupled with conditions such as diabetes [35], have earmarked these urologic conditions as important health issues also [35][36][37].
Context of chronic kidney disease in Australia
In Australia, the three major causes for ESKD since 2005 have been diabetes, glomerulonephritis (diseases where there is inflammation of the glomeruli) and renovascular disease/hypertension [4][38][39][40]. Abnormal lipid metabolism, proteinuria, sleep apnoea, mineral and bone disorder, anaemia, dietary protein restriction, poor nutrition, uraemia, acidosis, hyperkalaemia, restless legs, and depression are some of the other associated problems with impairment in kidney function [29]. The risk of such complications progressively increases as kidney function declines [28].
Factors contributing to kidney disease among Indigenous Australians
Factors contributing to kidney disease among Indigenous people are complex. They reflect a combination of broad historical, social, cultural, and economic factors, as well as the more commonly described proximal biomedical risk factors. The importance of historical, psychosocial and socioeconomic aspects is recognised, but it is beyond the scope of this review to address these factors in any great detail. It is also beyond the scope of the review to address comorbidity with diseases such as diabetes, which is recognised as an important contributing risk factor. As a result, the main risk factors addressed in this review are behavioural and biomedical risk factors.
Historical and socioeconomic context
The current health disadvantages experienced by Indigenous people can be linked to social disadvantages and should be viewed within a broad historical context [41][42]. The kidney health of Indigenous people has been affected by changes to their once active lifestyles and roles, brought about by displacement and colonisation by European settlers [43][44][45][46]. The reduction in activity levels of Indigenous people over time, coupled with poor nutrition resulting from displacement by Europeans, has become embedded in the social foundations and reflected in the social determinants of health [47]. This has played a part in the development of kidney disease and other chronic conditions such as diabetes over time, particularly in the later part of the 20th Century [45].
In addition to the modifiable risk factors (i.e. diet, obesity) for kidney health and other chronic conditions impacted by changes to the roles of Indigenous people [43][45][48], inadequate social and economic circumstances also underlie the generally poor health status of many Indigenous people and contribute to the high rates of kidney and urinary tract disorders in many Indigenous communities. Factors relating to the health-care system and government policies include limited access to primary and other medical care [49][50]. Sub-standard living conditions, inadequate environmental sanitation and poverty also play major contributing roles [7][50]. In Indigenous communities, prevention, control and management of kidney disease and associated disorders will depend not only on effective, acceptable treatment, but also on preventive action to address the poor socioeconomic conditions that underlie these conditions [51][52][53][54][55].
Behavioural and biomedical risk factors
The factors contributing to kidney disease among Indigenous people are generally attributed to various combinations of multiple risk factors including: repeated infections, high blood pressure, obesity [7][56], low birth weight [57][58], infant malnutrition [59][60][61], engagement in high risk behaviours that can adversely affect health (e.g. poor diet, low activity levels, alcohol and tobacco use) [1][2][50] and diabetes [2][4]. These conditions are quite common among Indigenous people, particularly Indigenous women, and contribute to high rates of kidney disease [2][23][56]. Given the modifiable nature of a majority of these factors, efforts to minimise these risks can help to reduce the prevalence of kidney disease and its associated mortality.
Extent of CKD and end-stage kidney disease (ESKD) among Indigenous people
Incidence and prevalence
As noted previously, the level of CKD is higher among Aboriginal and Torres Strait Islander people than among other Australians [2][3][4][5][6]. With no large-scale Indigenous biomedical survey available, it is necessary to rely on self-reported survey data as well as information from community-based reports and screening programs to estimate the prevalence of CKD among Indigenous people [10][11][12][13][14][15][16][17][18][19][20][28]. The most recent self-reported information available is from the 2004-05 National Aboriginal and Torres Strait Islander Health Survey (NATSIHS) with comparable data for non-Indigenous people available from the 2004-05 National Health Survey (NHS) [1][27][28][62].
In 2004-2005, kidney disease was reported by 2% of Indigenous people overall1 [1][2]. After age-adjustment, kidney disease was ten times more common among Indigenous people than among non-Indigenous people [1][2][27]. The prevalence of kidney disease for Indigenous people increased with age, from less than 1% in the 0-14 age group to 7% for Indigenous people aged 55 years and over (see Figure 1) [1]. Except for the 0-14 years age group, kidney disease was much more common among Indigenous people than among non-Indigenous people across all age groups. The level of kidney disease reported in the 2004-2005 NATSIHS was nearly twice that reported in 2001 [1][2][27].
Figure 1. Prevalence of kidney disease, by Indigenous status and age, Australia 2004-2005
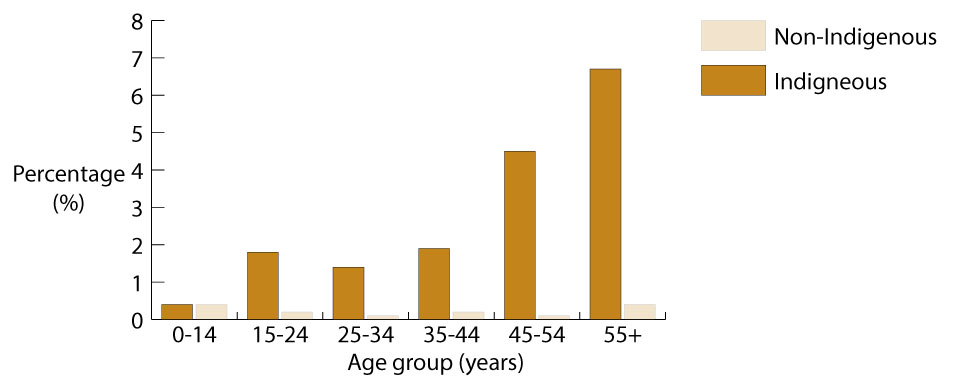
Source: [1].
Detailed information about CKD is only available from periodic health surveys, but progression to ESKD is reported routinely to the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA), which collates the data and produces annual surveillance reports [4][7][63]. These reports reveal that the overall incidence of ESKD is consistently significantly higher among Indigenous people than among non-Indigenous people [3][4].
A total of 675 Indigenous people were newly identified with ESKD between 2007 and 2009 – the age-standardised notification rate of 960 per 1,000,000 population for Indigenous people was almost 10 times the rate of 97 per 1,000,000 for non-Indigenous people (Table 1) (Derived from [64][65][66][67]). Incidence rates of ESKD were higher for Indigenous people than for non-Indigenous people in all states and territories, with the highest rates recorded for Indigenous people living in the NT (1,594 per 1,000,000), WA (1,194 per 1,000,000), and SA (876 per 1,000,000).
Table 1: Incidence and age-standardised incidence rates for end-stage kidney disease, by Indigenous status, and Indigenous:non-Indigenous rate ratios, selected jurisdictions, Australia, 2007-2009
| Jurisdiction | Indigenous | Non-Indigenous | Rate ratio | ||
|---|---|---|---|---|---|
| Number | Rate | Number | Rate | ||
| Source: Derived from [64][65][66][67]. | |||||
Notes:
|
|||||
| NSW | 80 | 283 | 2,200 | 98 | 2.9 |
| Vic | 19 | 415 | 1,601 | 101 | 4.1 |
| Qld | 167 | 637 | 1,318 | 106 | 6.0 |
| WA | 156 | 1,194 | 605 | 97 | 12.4 |
| SA | 44 | 876 | 503 | 107 | 8.2 |
| NT | 201 | 1,594 | 36 | 89 | 18.0 |
| Australia | 675 | 960 | 6,574 | 97 | 9.9 |
More than three-fifths (63%) of Indigenous people newly registered with ANZDATA in the period 2007-2009 were aged less than 55 years, compared with less than one-third (30%) of non-Indigenous people registered (Derived from [64][65][66][67]) (Table 2). Age-specific notification rates were higher for Indigenous people than for non-Indigenous people across all age groups except those aged 0-14 years. Rate ratios were particularly high for people aged 35-44 years (12.6) and 45-54 years (14.3).
Table 2: Incidence and age-standardised incidence rates of end-stage kidney disease, by Indigenous status and age group, and Indigenous:non-Indigenous rate ratios, Australia, 2007-2009
| Age (years) | Indigenous | Non-Indigenous | Rate ratio | ||
|---|---|---|---|---|---|
| Number | Rate | Number | Rate | ||
| Source: Derived from [64][65][66][67]. | |||||
Notes:
|
|||||
| 0-14 | 1 | 2 | 83 | 7 | 0.2 |
| 15-24 | 12 | 37 | 155 | 18 | 2.1 |
| 25-34 | 48 | 214 | 313 | 36 | 5.9 |
| 35-44 | 143 | 703 | 507 | 56 | 12.6 |
| 45-54 | 222 | 1,528 | 932 | 107 | 14.3 |
| 55-64 | 183 | 2,239 | 1,360 | 190 | 11.8 |
| 65-74 | 59 | 1,670 | 1,667 | 375 | 4.5 |
| 75+ | 7 | 438 | 1,557 | 390 | 1.1 |
| All ages | 675 | 960 | 6,574 | 97 | 9.9 |
The high rates of ESKD are a major public health problem for Indigenous people, particularly those living in remote parts of the country. In 2006-2008, the incidence of ESKD varied greatly with remoteness [5]. The incidence of ESKD for Indigenous people was especially high in remote and very remote areas of Australia with rates almost 18 times and 20 times those of their non-Indigenous counterparts (Table 3).
Table 3: Standardised incidence rates of end-stage kidney disease, by Indigenous status, rate ratios, and remoteness, 2006-2008
| Remoteness | Indigenous (No. per 100,000) | Non-Indigenous (No. per 100,000) | Rate ratio |
|---|---|---|---|
| Source: Derived from [5]. | |||
Notes:
|
|||
| Major cities | 40 | 11 | 3.6 |
| Inner regional | 40 | 9 | 4.3 |
| Outer regional | 98 | 9 | 11.3 |
| Remote | 149 | 8 | 17.7 |
| Very remote | 168 | 9 | 19.9 |
| Australia | 80 | 10 | 8.0 |
The full extent of ESKD among Indigenous people is reflected by the far greater proportion of Indigenous people reliant on dialysis for KRT compared with their non-Indigenous counterparts [4]. As at 31 December 2008, of all Indigenous ESKD patients registered with ANZDATA, 88% relied on dialysis compared with 55% of non-Indigenous Australians [5]. Only 12% of Indigenous people had received a kidney transplant, compared with 45% of their non-Indigenous counterparts.
Hospitalisation
A recent report on hospitalisation confirms that Indigenous people are admitted to hospital much more frequently for CKD and ESKD than are non-Indigenous Australians [23]. In 2009-10, care involving dialysis was the most common reason for the hospitalisation (Box 3) of Indigenous people living in NSW, Vic, Qld, WA, SA and the NT, with Indigenous people hospitalised 11 times more often than were their non-Indigenous counterparts [24]. In 2007-08, Indigenous females had the highest rate of dialysis hospitalisations, almost 15 times that of other females, and Indigenous males were 8 times more likely to be hospitalised for dialysis than were other males [23]. For the same period, the hospitalisation rate of Indigenous people for the procedure of dialysis was more than 12 times that of non-Indigenous people [68].
Box 3. Information about hospitalisation
Hospitalisation data are not a good reflection of the level of CKD in the community, but can give some indication of the impact of the disease as well as provide information on who is accessing services. Dialysis treatment is the most common reason for hospitalisation in Australia, with patients on dialysis needing to attend a hospital or satellite centre (attached to a hospital) three times a week for treatment [23][49]. A person who has frequent admissions for the same disease (such as dialysis) is counted multiple times, so it is important to separate hospitalisation rates for dialysis from rates for other conditions [49].
In 2007-08, the most recent period for which this information is available, Indigenous people living in NSW, Vic, Qld, WA, SA and the NT were five times more likely than their non-Indigenous counterparts to be hospitalised for principal or additional CKD diagnoses other than care for dialysis [23]. Hospitalisation rates for CKD as both principal diagnosis and for additional diagnoses were between five and seven times higher for Indigenous females than for other females2.
Mortality
In 2004-2008, the age-standardised death rate for kidney disease among Indigenous people living in NSW, Qld, SA WA and the NT was 5.1 times the rate for their non-Indigenous counterparts3 [56]. The death rates for kidney disease for Indigenous people living in NSW, Qld, WA, SA and the NT, increased by 102% over the eight-year period 2001-2008, compared with a 23% increase for their non-Indigenous counterparts [5].
Earlier data from 2001-2005 reveal that death rates involving CKD were especially high after the age of 25 years for both Indigenous males and females compared with the rates for their non-Indigenous counterparts [27]. In the 45-54 years age group, Indigenous males were 31 times more likely than non-Indigenous males to die from CKD. For the same age group, Indigenous females were 51 times more likely to die from CKD than were non-Indigenous females.
Deaths involving CKD can occur in the context of other chronic conditions, so published death rates for CKD underestimate its contribution to mortality [7][69]. For example, a common underlying cause of death for Indigenous Australians living in NSW, Qld, WA, SA and the NT in 2003-2007 where CKD was an associated cause (Box 4) was diabetes (28%), but for non-Indigenous Australians this was the equal least common underlying cause of death (8%) [7].
Box 4. Underlying and associated causes of death
Cause of death coding provides for classification according to whether a condition was an underlying or associated cause of death [3][7]. This means that CKD can be coded as the underlying cause (i.e. either led directly to an individual’s death) or coded as an associated cause (i.e. contributed to the death but was not related to the condition causing it) [7].
Table 4: Underlying cause of death where CKD was an associated cause, by underlying causes and Indigenous status, NSW, Qld, WA, SA and NT, 2003-2007
| Underlying cause of death (ICD-10 codes) | Indigenous | Non-Indigenous | ||
|---|---|---|---|---|
| Number | Per cent | Number | Per cent | |
| Source: [7]. | ||||
Notes:
|
||||
| Cardiovascular diseases (100-199) | 326 | 30 | 13,816 | 44 |
| Diabetes (E10-E14) | 303 | 28 | 2,432 | 8 |
| Neoplasms (C00-D48) | 107 | 10 | 6,062 | 19 |
| Respiratory diseases (J00-J99) | 88 | 8 | 2,636 | 8 |
| Other (balance) | 263 | 24 | 6,634 | 21 |
| Total | 1,087 | 100 | 31,580 | 100 |
An analysis of deaths from kidney disease for the period 2002-2005 found that the age- standardised rate for Indigenous people living in remote areas was higher than the rates for those living in outer regional and very remote areas (7.9 per 1,000 population compared with 4.6 and 4.8 respectively) [70]. The higher rate for remote areas than for very remote areas is contrary to expectations. The authors raised the possibility that this could be at least partly due to a better social environment, increased physical activity, healthier diet and lower consumption of alcohol and other drugs in very remote areas compared with remote areas. An alternative explanation is that the difference in death rates could be due to selection bias; that is, people from very remote areas tend to move to population centres for better access to health services for themselves or sick family members.
Other kidney health disorders
Glomerulonephritis
Like the broad terms ‘kidney disease’ and ‘renal disorder’, ‘glomerulonephritis’ is a collective term that refers to a number of pathologies that cause inflammation of the glomerulus and subsequent damage to the filtration process of the kidney [35][43]. Of the various conditions covered by the term, acute post-streptococcal glomerulonephritis (APSGN; see Box 5) is the most important contributor to high rates of chronic kidney disease and continues to pose a significant public health problem in developing countries and among Indigenous populations of the developed world [33][35].
Box 5. Acute post-streptococcal glomerulonephritis (APSGN)
APSGN is a potentially serious, non-suppurative condition that occurs two to three weeks after skin or throat infection with some strains of group A streptococcus (GAS) bacteria [34][71]. Occasionally, infections with groups C or G streptococcus may also cause APSGN [34]. ASPGN can cause high blood pressure, haematuria with reduced serum complement levels, albuminuria and oedema, and is often complicated with acute kidney failure [34][72]. The usual period between infection and development of APSGN is 1-4 weeks with an average of 10 days, but this may be longer (3-6 weeks) after streptococcal skin infections or shorter (1-2 weeks) after streptococcal throat infections [73][74].
Extent of acute post-streptococcal glomerulonephritis (APSGN) among Indigenous people
Incidence and prevalence
APSGN has been uncommon among affluent populations of developed countries since the mid-1900s due mainly to the improvements in living conditions and later because of the availability of antibiotic therapy and improved access to health care [33]. APSGN is relatively uncommon in the general Australian population, but poses a significant public health problem in tropical Australia where pyoderma, skin infections and scabies are endemic [34][72][75][76][77][78][79][80] with up to 50% of the children infested with scabies [52][81][82]. The incidence of APSGN in the NT is among the highest in the world with a rate in Indigenous children under 15 years of 94 per 100,000 population reported for the period 1991 to 2008 [34][83].
Most cases of APSGN follow skin infections rather than throat infections because skin infections are the most pervasive problem [34][72]; children aged between 12 months and 17 years are predominantly affected [34]. Secondary infection of scabies with GAS, which occurs in 50-70% of cases, is the principal cause of APSGN [84]. APSGN outbreaks continue to occur in northern Australia with a yearly peak between April and June of sporadic cases and small clusters; larger and more widespread outbreaks occur around every five years across the Top End [34][75].
Results of studies on the contribution of post-streptococcal glomerulonephritis (PSGN) to chronic kidney disease vary, but recent research [14][53][84][85], and previous research undertaken in isolated Indigenous communities experiencing outbreaks of APSGN in 1980 [86] and 1987 [87] suggest that a history of APSGN in childhood is a risk factor for subsequent kidney dysfunction [14][83][88]. Findings from an early study, which used the albumin to creatinine ratio (a sensitive early marker of kidney damage) as its main outcome measure, suggest that about one-quarter of cases of overt albuminuria may be attributable to APSGN in childhood [88]. Albuminuria has since been shown to mark early chronic kidney disease in Aboriginal and Torres Strait Islander people [11][12][14][89][90][91]. Detailed consideration of the early markers of kidney disease is beyond the scope of this review, but current available data suggest that albuminuria may be a better prognostic marker of kidney disease than a low estimated glomerular filtration rate, which may be inaccurate in the Indigenous population based on comparisons made between an urban Indigenous population and those from the general Australian adult population [91].
APSGN outbreaks in Indigenous communities
Outbreak investigations and surveillance studies provide some indication of the extent of APSGN within Indigenous communities [72][73][75][76][79][83][92] but methodological difficulties surrounding the identification of cases and the estimation of rates suggest that the true incidence of APSGN is likely to be higher than these estimates [83][93]. Identification issues include: lack of a standardised case definition; limitations and logistical difficulties associated with diagnosing the condition in isolated communities [33][93]; and the high proportion of sub-clinical cases of disease [33][34].
In the last decade, Indigenous children with skin infections during APSGN outbreaks were five times more at risk to develop ASPGN than were their non-Indigenous counterparts [80]. In 2008, an outbreak was recorded in the NT where a 5 year old child first presented with GAS and subsequently APSGN. In the following two days, two more cases were found. A complete screening of school-aged children (under 15 years) revealed that 36% of the screened children presented with skin sores and 12.5% presented with scabies, but no other case of APSGN was found [75]. In 2007, prospective surveillance investigating the epidemiology of GAS in three remote Aboriginal communities in the NT found that inadequate health awareness, crowded household conditions and population mobility were important driving forces behind GAS dissemination, with household acquisition commonly transmitted via 5-9 year olds [94][95]. Responses to APSGN outbreaks have seen improvements in screening, but Indigenous communities in the NT continue to experience epidemics of APSGN around every 5-7 years with the last known outbreak occurring in 2008 [73].
Numerous outbreaks in the NT have received rigorous investigation and sound documentation since the early 1980s [73][75][76][79][80][83][86][87][92][95][96][97][98], but, until more recently, epidemics have been less frequently documented in other areas of northern Australia. Prior to 1993, not a single epidemic had been reported in far north Qld for almost 20 years, but 58 cases (10% of all children screened) were diagnosed among Indigenous children from three remote communities in that year [96] and in 2006, an outbreak of ASPGN was recorded in the Lockhart River community in far north Qld [72]. From over 87 children screened, 46% were infected by scabies. In a four month period, 11 cases of ASPGN were confirmed. More recently, a retrospective review of the clinical records of all cases of invasive group A streptococcal (iGAS) disease notified to Qld health authorities during the 5-year period 2004-2009 revealed that there was marked underreporting in Qld [77]. Even with reports across different districts differing markedly and a large number of isolates not reported, rates of invasive disease were higher in the Indigenous population than in the non-Indigenous population. As revealed by previous research, rates of iGAS were especially high in infants, with the incidence 10 times higher among Indigenous infants than among their non-Indigenous counterparts (123 per 100,000 compared with 12 per 100,000).
Mortality and morbidity
The clinical course of APSGN has been described usually as mild, with complete recovery within days or weeks [34][86][88][99]. The long-term outlook has generally been regarded as excellent [88], but, despite the apparently benign clinical profile, APSGN has also been associated with significant morbidity, hospitalisation and occasional mortality [93][97]. Recent information on mortality and morbidity associated with APSGN are not available, but it is known that APSGN may occasionally cause acute kidney failure and that hypertension is the most frequent major complication [34].
Earlier research investigating the morbidity associated with APSGN in far north Qld reported that of 100 identified cases (of which 96 were Indigenous), 72 were admitted to hospital where they remained an average of 8.5 days (range: 3-30 days) [93][97]. Major complications included severe hypertension (40 per cent of all cases), acute kidney failure (18 per cent of all cases), fluid overload/pulmonary oedema (12 per cent of all cases) and bacteraemia (6 per cent of all cases). One child died, another developed pneumococcal pericarditis and a purulent effusion and required surgery, and a young pregnant woman underwent dialysis to manage acute kidney failure – all were Indigenous [97].
Factors contributing to high levels of APSGN in northern Australia
Humid climatic conditions in tropical northern Australia, the ubiquity of scabies and skin sores, and the relatively poor social and economic circumstances of many Indigenous people as identified elsewhere in this review, all contribute to the high rates of APSGN [34][52][81][82] [35][79][100]. In communities with high levels of scabies and skin sores, new strains spread very quickly with transmission facilitated by overcrowded living conditions and infrastructure problems in many remote communities [78][82]. The ease with which new strains of GAS are transmitted in families and communities is reflected in the rapid spread of APSGN [34]. Limited access to medical care, poor personal hygiene and inadequate environmental sanitation are also major contributing factors to the spread of the disease [34][78][82].
Prevention and management of ASPGN
There is no vaccine for GAS and no simple treatment for APSGN. In the absence of interventions, new cases can continue for several months [34]. Attempts to control APSGN outbreaks (Box 6) in Indigenous communities have frequently involved trying to stop the transmission of the bacteria by mass administration of parenteral penicillin to all children with any sign of skin sores [34][72][92][96][99], and sometimes regardless of whether they present with skin sores [76][79]. The targeted treatment of children with skin sores and household contacts has shown to be an effective intervention with the need for fewer injections (the injection is painful and reactions may occur) and a less labour-intensive response (that is correspondingly less likely to compromise routine services or miss high-risk children) [79][99]. Past trials employing more sensitive outbreak case definitions (‘sub-clinical’ cases now referred to as ‘probable cases’ and ‘clinical cases’ referred to as ‘possible cases’) have also resulted in an improvement in the screening of high risk communities [79].
Box 6. Current guidelines for the control of APSGN
Following the recording of an outbreak of APSGN, communities are advised to follow the guidelines for its control provided by the NT Centre for Disease Control (CDC) [34]. Currently, the guidelines define a community outbreak as either:
- two unrelated cases, either probable or confirmed that meet the clinical definition of APSGN (without laboratory confirmation) with onset within a week or each other, or
- one confirmed case and two probable cases in one month of each other that are not epidemiologically linked as a family, household or close contact.
Guidelines include community education and screening of all children up to the age of 15 years for skin sores, scabies and oedema. Management of infection currently involves hospital admission for major complications, coupled with the use of antibiotics to inhibit spread of the disease. If oedema is present, then the child is strongly advised to be assessed for manifestations of APSGN [34][52].
A recent community screen in April 2011 reported that 57% of targeted children were screened [73]. The successful access to the community largely resulted from the initial groundwork undertaken by the local health service, which facilitated community engagement beforehand as well as the expertise of the local Aboriginal health workers in identifying children and families. The use of ‘high quality’ rewards (in the case of this screening it was a plastic Sticky Hand developed by the Environmental Health Program) to attract more children to screening and treatment was also noted as a successful strategy to assist with the engagement of children about to receive injections.
In summary, good primary health care has been recognised as vital in the recognition and referral of APSGN, as early recognition of outbreaks and timely treatment is crucial in preventing the spread of GAS in the community and possible irreversible loss of kidney function [34][74]. The coexistence of high rates of GAS infections, APSGN and ESKD in northern Australia has raised the question of whether childhood APSGN could be linked with chronic kidney disease in later life [53][75][85]. With the aetiological link between scabies, streptococcal skin infections and outbreaks of APSGN well recognised [34][52][101], interventions for sustainable long-term improvements in skin health and the integration of environmental health, housing and hygiene programs with such interventions, are an essential part of the prevention, management and treatment of APSGN outbreaks [75][79][101][102][103][104]. An improvement in education, sanitation, and access to treatment is also a crucial step toward decreasing the incidence of glomerular disease [9][35]. Secondary and tertiary prevention depends on improved surveillance, screening to identify cases, and penicillin prophylaxis to eradicate streptococcal carriage and stem the spread of epidemic disease [52][96]. Prevention at this level will not only reduce rates of APSGN but may also contribute to a reduction in the incidence of ESKD [51][52][88].
Disorders of the urinary tract
Disorders of the urinary tract (specifically disorders of the bladder, ureters and urethra) generally present as clinically mild cases, but they have the potential to cause considerable morbidity, and may, on occasion, lead to severe kidney disease, including ESKD [35][36]. The potentially serious consequences associated with urinary tract disorders, coupled with their prevalence (particularly among women), highlight the public health significance of these conditions. Urinary tract infections (UTIs) (Box 7) are generally bacterial in origin, but, fungi, parasites and adenoviruses may also cause infection [36].
Box 7. Urinary tract infections (UTIs)
Most UTIs occur in the lower urinary tract as cystitis (in the bladder) and urethritis (in the urethra) [36][103]. Pyelonephritis, an upper UTI, is very serious as it may damage the kidneys. The urinary tract is normally free of bacteria and other micro-organisms, but these organisms can get into the bladder by coming up the urethra from the outside; sometimes they can enter from the kidneys or bloodstream [103]. Once in the bladder, bacteria can multiply very rapidly in warm urine. A UTI may be present despite few physical symptoms, but lower UTIs are typically associated with frequent, painful urination, and tenderness in the lower pelvic area [36]. Upper UTIs are associated with a range of clinical features, most commonly fever, back or loin pain, and chills [103]. Single episodes of UTI rarely have serious consequences, but recurrent or persistent infections may cause kidney damage when coupled with conditions such as diabetes [35]. Lower UTIs are easily treated, generally by a single dose or short course of antibiotics [36][103]. Upper UTIs usually require hospitalisation and administration of intravenous antibiotics. Antibiotics may also be administered to prevent recurrent infection.
UTIs are one of the most common reasons for people visiting a doctor about an infection [36]. Females are much more likely than males to contract UTIs, and other risk factors include age and sexual activity [35][105]. In Australia, about one in three adult women and one in 20 men will have a UTI in their lifetime. Women tend to be more susceptible to UTIs for several reasons, including the length of their urethra; the urethra is much shorter in females than in males, making it easier for microorganisms to travel to the bladder. Other factors include changes in hormonal levels (women are more likely to get an infection during certain times in their menstrual cycle, particularly just before a period and are also more susceptible during pregnancy). In women, the tissues of the urethra and bladder become thinner and drier with age, as well as after menopause or a hysterectomy: this also increases susceptibility to UTI [36][103].
Extent of UTIs among Indigenous people
The health impact of diseases of the urinary tract upon Indigenous people has received very little attention in recent years, but information from the 1980s suggests that the pattern of UTI exhibited among Indigenous people tends to differ from that observed among non-Indigenous people [105]. Epidemiological and anecdotal evidence from the 1980s indicates that UTIs were particularly common among Indigenous people [105][106]. A hospital-based study in Darwin found that Indigenous men and women had higher rates overall of UTI than did their non-Indigenous counterparts, but age-specific rates were greater among non-Indigenous people after the age of 60 years [105]. With the exception of children in their first year of life, UTIs occurred far more frequently among Indigenous females, with consistently high rates of infection until around 60 years of age. Indigenous males, on the other hand, were at greatest risk during infancy. Unfortunately, easily treated UTIs often remained undetected, particularly in Indigenous children, which increased the individual’s risk of developing more serious kidney disease [106]. Inadequate living conditions and poor environmental standards are thought to contribute to the high levels of UTI observed in some Indigenous communities [35][106].
Other data reveal that Indigenous women are more than twice as likely as non-Indigenous women to have a UTI during pregnancy [107][108]. A retrospective audit of medical records from rural and remote primary health care clinics in the NT of 268 pregnant women who gave birth in 2002 or 2003 revealed that 75% had at least one abnormal urinalysis during pregnancy (620 episodes were recorded overall) [107]. This is consistent with findings from earlier research that found almost 30% of Indigenous women sampled had a UTI during pregnancy, and 11% had an infection at the time of delivery [109]. Evidence from these studies suggests that screening, treatment and follow-up of UTIs among Indigenous people was often inadequate [106][107][108][109]. The occurrence of pyelonephritis (acute UTI that has reached the pelvis of the kidney) in women who had non-treated asymptomatic bacteriuria during pregnancy highlights the importance of screening, particularly for women living in rural and remote Indigenous communities.
Urolithiasis (kidney stones)
Urolithiasis refers to the formation of one or more pebble-like masses (commonly referred to as calculi or stones) in the kidney or urinary tract [31][32]. It is not a common public health problem within affluent populations in developed countries, but its prevalence and unusual presentation among some Indigenous children justifies its coverage here [37].
Extent of urolithiasis among Indigenous people
Very little attention has been directed at urolithiasis among Indigenous people since the 1990s, but the presence of a unique pattern among Indigenous children has been confirmed recently [37]. The findings of this study of Indigenous children presenting to the Darwin Hospital were very similar to those reported in the 1980s and 1990s [110][111][112][113][114][115][116].
The Darwin study found that Indigenous children living in remote areas of tropical and desert Australia have an unusual pattern of kidney stones [37]. Stones form early in life: the median age of presentation was 24 months with some stones recognised as early as 8 months of age. This pattern contrasts with that reported among non-Indigenous people and other populations of the developed world where the incidence is much higher among adults than children [37][110][115].
The recent research [37] is consistent with the reports from the 1980s and 1990s: Indigenous children with urolithiasis tend to come from desert regions of Australia, are more likely to be male, and are frequently less than 3 years of age [110][111][112][113][115]. Urolithiasis is rare among Indigenous children living in urban areas [37]. Children with stones commonly present with, or have a history of, failure to thrive, UTI, and/or recurrent infectious disease (particularly diarrhoea) [37][110][111][112][113][115]. The stones found in Indigenous children are rarely associated with anatomical or metabolic disorders and are commonly located in the upper urinary tract [110][111][113][115]. They are composed primarily of uric acid, and oxalate [110][111][112][113], similar to the ‘endemic’ stones typically found in paediatric populations from developing regions of the world where the disorder is prevalent [110].
The formation of calculi in non-Indigenous Australian children is uncommon [37][110][112]. When urolithiasis does occur in non-Indigenous children, it presents at a later age (usually mid to late childhood) and is generally attributable to a malformation of the urinary tract or a metabolic disorder [110][112]. A comprehensive review of patient records collected between 1972 and 1986 from the major paediatric referral hospital in WA reported that the number of Indigenous children presenting with urolithiasis was more than double that of non-Indigenous children [110]. A review of patients with urinary tract calculi admitted to the Urology Unit of the Adelaide Children’s Hospital between 1978 and 1987 estimated that 0.34% of Indigenous children under 10 years of age (based on 1981 census figures) suffered from the disease [112].
Factors contributing to urolithiasis among Indigenous people
Acidic stones are produced when there is a loss of alkaline bowel content (as a result of diarrhoea for example) [37]. The high rates of urolithiasis observed among some Indigenous children have been attributed to dietary factors, dehydration, endemic diarrhoea, recurrent infectious disease [37][110][111][112][113][114][115], hot, dry environmental conditions [110][111] and poor water quality [114]. These risk factors are intimately related to the familiar socioeconomic risk factors that underlie the high burden of disease suffered by Indigenous people generally.
Management and treatment of urolithiasis
Some stones appear to resolve spontaneously, but others might require surgical removal to avoid the risk of kidney damage [37]. The specific causes of kidney stones should be treated appropriately, but general treatment includes increased fluid intake (i.e. drinking water which is especially important for children during the weaning period and in for those warm climates), limited daily salt intake, moderate animal protein intake and medical treatment with alkali and thiazides (including sodium bicarbonate or potassium citrate [35][37]. Attention must be directed to the long-term public health implications and the need for preventive measures [112]. Housing, water and waste disposal systems are inadequate in many Indigenous communities and increase the risk of urolithiasis. Improvements in environmental conditions, specifically the provision of adequate drinking water and the eradication of poor living conditions, are therefore essential to reduce the incidence of kidney stones [37][114].
Management and treatment of CKD and ESKD
Kidney disease is expensive to treat and has a marked impact on the quality of life of those who suffer from the disease and of those who care for them [8][27]. Medical intervention is necessary to prevent deaths among individuals with CKD and ESKD. The current treatment option for ESKD involves KRT including dialysis (either haemodialysis (HD) or peritoneal dialysis (PD) (Box 8) and transplantation [9][29]. The aim of treatment for CKD (prior to reaching ESKD) is to delay progression of the disease. If CKD progresses to ESKD, KRT becomes necessary with the aim of providing ongoing quality and quantity of life rather than focusing the prevention of further deterioration of kidney function [9]. KRT cannot cure failing kidneys, but dialysis can enable survival. A further aim of treatment is to prevent added complications, such as cardiovascular issues [28][29]. Therefore, management of CKD also involves lifestyle modifications including: cessation of smoking; maintaining BMI below or equal to 25 kg/m2; engagement in regular physical activity; reducing alcohol and drug consumption; and maintaining a low salt diet [29].
Box 8. Dialysis: haemodialysis (HD) and peritoneal dialysis (PD)
Regular dialysis mechanically filters the blood to help maintain the functions normally performed by the kidneys [7][9]. There are two possible dialysis treatment options; haemodialysis (HD) and peritoneal dialysis (PD) [3][7][117]. Both treatment options aim to replace part of the (damaged) kidney’s function and both can be conducted from home and by the patient themselves [7][117].
Haemodialysis (HD)
In HD, blood is diverted from the body to a dialysis machine where it is filtered before being returned to the body [7][9]. As HD requires a costly machine to assist in the filtration of fluids outside the body [55], it is conducted at various locations, including hospital or dialysis units (located in tertiary hospitals), satellite centres (facilities linked with a tertiary centre renal unit) or managed carefully in the home [7][118]. The limited availability of such services outside of population centres, means that many Indigenous people are often forced to stay away from their land and families for long periods of time [55][118][119][120] and can mean that they die away from their people.
Peritoneal dialysis (PD)
In PD, the dialysis solution, containing a type of sugar that draws waste products and extra fluid out of the blood, is pumped into the abdomen and the blood filtered through the peritoneal membrane [9][55]. After a few hours the used solution containing waste and extra fluid is drained out of the body and replaced with fresh solution. The exchange takes about 30-45 minutes and can be undergone manually but requires dialysis exchanges 4-5 times daily; known as continuous ambulatory peritoneal dialysis (CAPD), or through the use of a machine which controls the timing of fluid exchanges while the patient sleeps; known as automated peritoneal dialysis (APD) [7][55][118]. Most people use PD at home, given its portability and convenience [7][118]. Both forms of treatment offer an alternative for people with CKD living in remote areas as it allows more independence and greater opportunity to return home more quickly [3][121].
Extent of dialysis among Indigenous people
A total of 187 Indigenous people commenced dialysis in Australia during 2009, a decrease from 249 in 2008 and 237 in 2007 [4]. In 2009, 1,174 prevalent dialysis patients in Australia were Indigenous; the level for Indigenous people was nearly five times that of their non-Indigenous counterparts (2,220 compared with 473 per 1,000,000 population) (Derived from [4]).
Based on information about hospitalisation, ‘care involving dialysis’ was most common for Indigenous people living in very remote areas (154 hospital separations per 1,000 population) [68]. Similarly, hospital separations relating to renal failure by socio-economic quintiles4 revealed that the ‘most disadvantaged’ quintile (representing the areas containing the 20% of the population with the least advantage/most disadvantage) had the highest separation rates dialysis. The residence in very remote areas of many Indigenous people requiring dialysis has important implications for how appropriate services are delivered.
Issues associated with relocation to undertake dialysis
When dialysis facilities are not available near to where the Indigenous patient lives, they are forced to move to regional centres or major cities to undertake dialysis. The lack of treatment available in remote areas and the limited availability of transplant facilities create geographical barriers to treatment with 78% of patients in remote areas having to relocate, compared with 39% of those who live in rural areas and 15% of urban Indigenous ESKD patients [6]. In recent years, research has revealed the significant biological, psychological, social and economic consequences to the forced relocation of Indigenous patients [122][123].
A study of Indigenous renal patients moving to Alice Springs from remote central Australian communities reported the socio-cultural alienation as extreme, debilitating and ultimately life threatening [124]. Recent research in WA reported similar findings for relocated Indigenous renal patients in that state [123]. The loneliness resulting from removal from family and land, including a sense of loss and disempowerment were considered worse than the illness (CKD) itself. Added to the stress of not being able to attend cultural events and obligations, the geographical isolation associated with treatment options often discourages Indigenous people from seeking treatment [6][123]. The impact of such issues is further highlighted by data reporting social causes as the second most important cause of death of Aboriginal and Torres Strait Islander people on dialysis (24%), after cardiac events (37%) [4].
In a bid to address poor treatment outcomes associated with relocation, efforts have been made to deliver self-care PD and HD dialysis services close to, or in, the home [125]. This means that Indigenous people remain in their own communities, with their own family and social supports, and the costs of re-housing and supporting relocated dialysis patients are avoided. However, challenges facing home HD in remote communities include being able to retain suitable dialysis partners/carers due to their requirements for attendance at cultural duties, suitable housing, changing social circumstances and communication problems (such as language barriers) [118][126]. In Queensland, three health zones have developed Renal Service Plans in recognition of Indigenous people as a priority population and in acknowledgement that many rural/remote areas of the state do not have ready access to hospital renal units or satellite units [127]. These plans include specific strategies to address kidney disease including health promotion, early detection, clinic management, kidney replacement services, workforce management and information support actions all specifically tailored to the needs of Indigenous people. Examples include the opening of the Mt Isa satellite service and the multi-user, self-care dialysis strategy established in the Toowoomba Renal Service. Satellite dialysis facilities are becoming more common in remote areas, but, in view of the low population density in many areas, access to treatment for people with ESKD is still an issue that requires dramatic changes in living circumstances [69][128]. Given ESKD relocation and treatment issues, it is considered good practice to allocate patients to the closest treatment facilities and to develop socio-culturally sensitive services for the specific needs of Indigenous patients.
In the search for alternative dialysis treatments, research has examined the use of Automated Wearable Artificial Kidneys (AWAK) [129]. Potential advantages proposed for this type of device include steady-state metabolic and fluid control, continuous regeneration of spent dialysate and the round-the-clock functionality of the device (mimicking the natural kidney). Such outcomes would allow patients to stay at home for their treatment and minimise interruption to their everyday activities. Despite AWAK’s potential to affect the wellbeing of CKD sufferers, especially Indigenous people in rural and remote areas, this type of treatment is still in a speculative stage of development.
Extent of HD among Indigenous people
Conducted in urban or regional clinics and hospitals, HD is the most common form of dialysis treatment for Indigenous people with ESKD [4][27][55][118][120]. In 2009, HD accounted for 81% of the new cases of Indigenous people commencing dialysis [4][40] and for the period 2007-08, hospitalisation data showed that Indigenous people were undergoing HD at 12.1 times the rate of non-Indigenous people5 [68]. In the same year, one in three hospital procedures for Indigenous peoples were for HD with recorded hospital separations higher in more remote areas.
A recent study comparing clinical outcomes and mortality rates of Indigenous people of Kimberley origin receiving HD treatment with other subsets of Indigenous HD patients and non-Indigenous Australians, revealed for the first time, similar mortality rates [130]. As a response to the new model of care for Kimberley patients undergoing HD in 2002, the study investigated outcomes for patients of Kimberley origin between January 2003 and December 2007, including patients receiving any form of KRT in any Australian location for that time period. An overall average of 70% of patients of Kimberley origin received HD treatment mainly provided by the Kimberley Satellite Dialysis Centre (KSDC), operated by an Aboriginal Community Controlled Health Service (ACCHS). The treatment was delivered at KSDC, at home in communities across the Kimberley, and at Derby Aboriginal Health Service. The study found that Indigenous people from the remote Kimberly region received HD treatment as good as elsewhere in the country. The authors felt that the excellent adherence of patients to care could be attributed to the delivery of quality services in culturally appropriate settings.
Extent of PD among Indigenous people
In 2009, the number of Indigenous people commencing peritoneal dialysis (PD) (35 patients) in Australia was less than in the previous two years [4]. By reducing the risks associated with relocation for HD, there are potential advantages of PD. However, as PD requires the patient to replace the dialysate bag several times a day with failure to change the bag resulting in life- threatening conditions, this places more responsibility on the patient to ensure correct management of the treatment [55]. Research has shown the correct management of PD treatment is impeded by various factors, such as infections and/or problems with the peritoneal dialysis catheter, home environment and child responsibility, constipation and treatment simply not suiting the individual [55]. Some anecdotal evidence also indicates that issues associated with changing the dialysate bag at home, such as facility issues with power and water, often leads to Indigenous people making the choice to undertake HD away from home. As previously discussed, this can create stress associated with having to relocate [123].
General issues associated with HD and PD
The multifaceted negative implications of HD and PD for Indigenous patients also reflect the differences between medical culture and that of Indigenous communities. Miscommunication between Indigenous patients and health care workers can occur because of differences in cultural and social beliefs on health, and in the understanding of the patient’s situation and ongoing treatment [43][131][132]. Such miscommunication is exacerbated for those Indigenous patients for whom English is a second language. Appropriate information and the associated understanding of the patient and their family are key for the patient – and their family and community – to make the important decisions about treatment choice [124][131][133].
Transplantation
Transplantation involves the implantation into a patient with ESKD of a kidney from either a living or recently deceased donor [7][9]. A recent systematic review of 110 international studies including a total of 1,961,904 participants with kidney failure revealed that transplantation was associated with substantial reductions in the risk of mortality and cardiovascular events [134]. It was also associated with clinically relevant improvements in quality of life, which improved further over time compared with treatment with different dialysis modalities. These findings confirm that kidney transplantation, when feasible, is the preferred therapeutic option for the management of CKD for patients and by health-care professionals [134][135]. Earlier studies revealed that the annual death rate of an age-matched population maintained by transplantation was reduced by about 80% beyond the first year compared with those remaining on dialysis [135][136]. The use of kidneys with no human leucocytes antigen (HLA) mismatches was associated with superior outcomes, but, unfortunately, completely matched kidneys account for a very small proportion of organs transplanted [137].
Extent of transplantation among Indigenous people
Transplantation is the optimal form of treatment for most ESKD patients, but there is a pronounced disparity between Indigenous people and non-Indigenous Australians for this treatment [117]. At the end of 2008, a far greater proportion of Indigenous people than non-Indigenous Australians were reliant on dialysis for KRT: only 12% of Indigenous people were living with a functioning transplant compared with 45% of other Australians6 [5]. Indigenous patients are both less likely than other Australians to receive a transplant and less likely to be wait-listed for a transplant [4][117][137]. Indigenous people receive transplants at approximately one-third the rate of other patients and those who do receive transplants wait longer for them [4].
In the four-year period 2006-2009, 2.1% of Indigenous people needing KRT received a transplant, compared with almost 11% of non-Indigenous people (Derived from [4]). For the same period, only 4% of all people on the waiting list were identified as Indigenous. Only 100 new transplant operations involved Indigenous recipients compared with 1,741 for non-Indigenous recipients. In most Australian states, the average wait for a kidney transplant from a deceased donor is about 4 years, and for some patients the wait is much longer [135]. Those Indigenous people who undergo transplantation usually need to travel large distances to reach medical centres [6][138].
Factors associated with access to kidney transplantation
Research investigating the reasons for the disparity between Indigenous ESKD patients and non-Indigenous patients receiving transplants (Box 9) reveals some key barriers to the accessibility of treatment [117]. System factors included: reduced likelihood of referral for transplant evaluation; failure to complete the comprehensive set of investigations required; and communication and education limitations. Individual factors included: greater likelihood of human leucocyte antigen (HLA) incompatibility; fear of their health status; refusal to leave land and community; higher rates of co-morbidities affecting acceptability for transplantation; and lower compliance to medical treatment as a result of communication problems and misunderstandings.
Box 9. Organ allocation in Australia
The allocation of organs, including kidneys, is a complex process that depends on a range of factors including medical need, urgency and the capacity of the recipient to benefit from the transplantation [139]. In Australia, allocation systems are underpinned by the principles of utility, equity and fairness. Kidneys matched by blood group and tissue compatibility are offered to the best suited recipient, regardless of where they live. There are only a few kidneys available for transplantation, so only patients deemed medically or otherwise appropriate are able to receive transplants. Patients are educated about the process to ensure informed decision making before being placed on the waiting list. When a donor organ becomes available or is identified, transplantation can take place [117].
A palliative care approach to the management and treatment of CKD and ESKD
Practices are being developed throughout Australia that contribute to the improvement of care, prevention and culturally appropriate management of CKD and ESKD [119][125][140][141][142][143][144][145][146][147]. Without the option of transplantation, dialysis is the only final stage treatment available for ESKD and, as such, it has the potential to be systematically coupled with palliative care units. For example; the Improving care for people with advanced chronic kidney disease: I’m OK project allocates Indigenous patients to a care coordinator who liaises with the different health professionals that the person sees [119]. The goal of the project is to reduce communication problems and enable close-to-home access to specialist health care in regional Aboriginal community controlled health services in the Northern Territory. The initiative offers a coordinated approach and contributes to the creation of strong links between the many different health staff who provide care to people with advanced CKD. The four-year long Palliative care for renal clients living in a remote setting project focuses on raising awareness of the palliative approach of the kidney health workforce and examines the palliative needs of clients with CKD [143]. The project aims to produce health resources and guidelines to accompany Indigenous renal patients through the end of their lives by providing support to make end-of-life decisions while also providing opportunity for health worker staff to embrace the palliative approach to care.
Prevention of ESKD
Kidney disease clearly has major medical and social implications for Indigenous people. The negative social consequences that accompany treatment and the high cost of tertiary level medical care illustrates the need for a comprehensive approach that addresses both the medical and socioeconomic dimensions of this growing problem [55][128].
Primary prevention of the social and economic conditions that underlie much of the Indigenous kidney health burden is a fundamental priority [53][54]. Secondary and tertiary measures, such as screening and pharmacological interventions, also promise to reduce the risk of serious kidney disease [148]. Screening for kidney disease has for some time been accomplished with simple, cheap and reliable techniques [61][149]. Such screening instruments may be readily incorporated within existing chronic disease screening protocols and coupled with interventions to modify the disease process [148].
An early example of a systematic treatment program implemented in the Tiwi Islands (off the coast of the NT) offering primary, secondary and tertiary prevention measures was designed to intervene in the disease process before individuals progress to ESKD. The program focused on vigorous blood pressure control and better metabolic management for people with diabetes and kidney disease, or with diabetes and high blood pressure and centred on the use of the long-acting angiotensin converting enzyme inhibitor, (ACEi) perindopril, an anti-hypertensive with cardiovascular and renal protective effects [150]. An early evaluation of the health outcomes of the program indicated that it had: slowed the progression of kidney disease; postponed kidney failure; reduced premature death; averted much cardiovascular morbidity; and diminished associated health care costs [21][151]. Progression to end-stage kidney failure had been reduced by one-half and there had also been a reduction in deaths from natural causes. The pattern of ESKD in the community had been reversed, with previously increasing rates of ESKD and natural death reduced [151].
The success of the program was attributed to a strong sense of community involvement and a partnership approach between the health workforce delivering the program and the community [152]. Specific facets of the program thought to have contributed to its success include:
- maximum involvement of local workers
- a community-based rather than clinic-based focus
- a collaborative, non-authoritarian approach
- involving participants in their own testing
- personalising health goals
- providing information not directives.
The program demonstrated that kidney disease can be easily diagnosed and its progression dramatically altered by available interventions [153]. It also showed that Indigenous people are interested in health issues and receptive to health messages; their willingness to participate enthusiastically and effectively in the long-term management of chronic disease led to demonstrable improvements in their kidney health [151]. Finally, the Tiwi model has shown that huge savings, in terms of both premature death and cost, are achievable if investments are made in community-based strategies for kidney disease [150]. It is estimated that the Tiwi program saved between $700,000 and $3.1 million in dialysis costs alone in its first three years of operation [153].
The need for comprehensive health care
In recent years, there has been significant research into the causes, consequences and management of ESKD in the Indigenous population [6][21][54][55][59][60][131][151][154][155][156][157]. A growing body of evidence demonstrates that some progress has been made [57], but it highlights, too, remaining gaps in the current health care approach. Consideration of the evidence as a whole provides guidance for the way forward, primarily indicating that Indigenous renal patients require, and deserve, effective, acceptable treatment. The current emphasis on complex, expensive, often problematic, hospital-based treatment should be balanced with comprehensive, community-based, preventive action to minimise the underlying causes of the problem [21][55][59][60][148].
The responsibility for reducing disparities in treatment rests primarily with the health care system and its providers [30][131][158][159]. This will require system-level changes, such as adequate funding for primary care, an adequate Indigenous health workforce, development of shared understandings about CKD, the use of high-quality and culturally appropriate educational resources, and improvements in the interface between primary care and specialist services. Prevention of the socioeconomic antecedents of kidney disease is inherent in such an approach, which calls for:
- sustained improvements in living and environmental conditions, education, infrastructure and health service [50][59][61]
- implementation of integrated, effective and well-resourced primary health care programs [21][59][69]
- primary health care measures to improve diet, control blood pressure and infections, maintain healthy adult weight, and to increase birth weight [30][50][59][61]
- systematic screening for early and established kidney disease [30][50][60][61][160][161]
- pharmacological intervention programs to slow disease development [35][60].
Evaluation is also a necessary component of any health program, and local ownership and management of health strategies for the Indigenous population is vitally important [55][148][162]. The added advantage of comprehensive action is that it will reduce not just kidney disease, but also other chronic and communicable conditions that underlie the much higher levels of mortality experienced by the Indigenous population [30][161][162]. It is within the capabilities of integrated socioeconomic and public health initiatives to quickly reduce kidney disease risk, and modify the existing disease profile, with potential savings in morbidity, mortality and health care costs [57][162].
Policies and strategies to improve kidney health
In Australia, the prevention and management of CKD and related kidney health issues is addressed more broadly under the banner of chronic diseases [163] and by way of strategies identifying areas for improvement in all aspects of CKD [69].
National strategies addressing CKD
The National chronic disease strategy (NCDS), released in 2006, includes information on CKD under the umbrella of chronic diseases [163]. The NCDS takes a holistic approach towards chronic diseases as these conditions often occur at the same time and their risk factors are similar. The strategy aims to better coordinate and manage chronic illnesses from prevention to palliation [163][164]. Further to this, the National chronic kidney disease strategy (NCKDS), proposed in 2006 by Kidney Health Australia, was released in response to the increasing burden of CKD in the Australian community [69]. Given the commonalities that exist between CKD and other conditions in the chronic non-communicable disease category (e.g. diabetes), the NCDS complements the NCKDS with synergies between the initiatives recognised within its framework and recommendations. Action areas across the strategies include: risk reduction, health promotion and illness prevention; early detection and early treatment and management; management of advanced CKD and self-management; and, more specific to CKD, priorities for dialysis, organ donation and transplantation [69][163].
With most of the underlying risk factors of CKD being primarily preventable, initiatives for risk reduction outlined by the NCDS and NCKDS include addressing the broad social determinants such as improvement of housing, education and employment opportunities, and the improvement, especially in remote areas, of affordable and healthy food choices and lifestyles [69][163]. Prevention and risk reduction approaches are applicable across the continuum of chronic disease care, aiming to prevent the diseases themselves, or their progression and associated complications and co-morbidities [163]. Early diagnosis offers the potential for both disease specific and non-specific interventions to slow disease progression. Even if progression cannot be slowed, patients who have been diagnosed early have better survival rates when commencing KRT [165]. Risk reduction seeks to prioritise the quality of life of the patient as well as the ability for self-management. Early treatment of CKD complications can also significantly improve the long-term patient outcomes [164]. In line with the current national strategy documents, self-management stems from the understanding that capacity building and training would enhance the ability of people to take more care for themselves (diminishing the impact of risk factors) and to control the health services in their communities (community-controlled services and increase of Aboriginal health workers) [163].
Recommendations and lessons learned in the delivery of services to improve kidney health
Addressing the needs of populations who are considered disadvantaged, including Aboriginal and Torres Strait Islander people, is of particular importance to both the NCDS and NCKDS [69][163]. Specifically, a key recommendation is the establishment of a national Aboriginal and Torres Strait Islander people chronic disease taskforce that transcends jurisdictional and community boundaries, as well as ensuring that all states/territories work towards providing equitable access to all forms of KRT for Indigenous peoples. In addition, the NCDS highlighted the importance of monitoring and improving data quality as part of the strategy towards reducing the burden of chronic disease [163]. In response to these needs, the National Centre for Monitoring Chronic Kidney Disease was established in 2007 at the Australian Institute of Health and Welfare (AIHW) [166]. In 2009, the Centre released its first publication An overview of chronic kidney disease in Australia 2009, which includes up-to-date national data on CKD [3]. Despite these developments, the various recommendations put forward by these initiatives have yet to be endorsed in the way of formal policies. Consequently, there is currently no comprehensive CKD strategy in Australia.
In 2010, the National Consumer Council of Kidney Health Australia7 released a position statement calling for the Australian Government to take action on the shortfall of services for CKD [128]. Similar in aims to the NCKDS [69], the position statement outlines changes to achieve optimal kidney health services and care in Australia. The main components of the position statement include: recognition of the extent of CKD as a major chronic condition; development of a national program to increase awareness and early detection of CKD; the funding and resourcing of the development and delivery of high quality information and education services in CKD; patient support programs and the training and promotion of these services in the community [128].
Recent work conducted by the Renal Division at the George Institute has involved several studies aimed at improving kidney health. One project – The central Australia renal study – commissioned by the Office for Aboriginal and Torres Strait Islander (OATSIH) within the Department of Health and Ageing (DoHA) – aimed to provide data and recommendations that would inform governments in the cross-jurisdictional region to make evidence based policy decisions that better meet the health and service needs of Aboriginal dialysis patients in the region [167]. Another project, commissioned by Kidney Health Australia, looking at the economic impact of the burden of CKD in Australia, aimed to establish the human and financial burden of CKD and to explore the cost-effectiveness of screening and intervention to prevent progression of disease [168]. Ongoing projects in the Division include the Improving access to kidney transplants (IMPAKT) project aimed at identifying Indigenous Australians’ barriers to accessing kidney transplantation, and proposing strategies to reduce disparities in Indigenous Australians’ access to transplantation [117][169]. In addition, findings from the Sharing the true stories (STTS), a longitudinal participatory action research (PAR) project (2001-05), provide support for solidifying policy in the kidney health arena [170]. Findings indicate that miscommunications and lack of shared understanding between Aboriginal client groups and health staff in renal and hospital services in the NT of Australia often limited patients’ opportunities and capacities to make informed choices about their health care. This suggests that, for there to be improvements in communication and health outcomes, fundamental change in the delivery of healthcare is required.
Other policies and one-off funding also continue to impact on the state of kidney health and related services in Australia. Under the umbrella of the Closing the gap strategy, which aims to reduce Indigenous disadvantages [171], the Council of Australian Governments (COAG) has shown commitment to reducing the factors that contribute to chronic disease [172][173]. Similar to the NCDS, COAG focuses on a preventive health approach through the strengthening of primary health care as well as focussing on sustainability and cultural appropriateness [174]. In addition, the Australian Government has funded specific initiatives to reduce the incidence and spread of kidney diseases including: grants into kidney disease research via the National Health and Medical Research Council (NHMRC); the construction of a renal dialysis unit at the North Lakes Health Precinct in Queensland; funding towards improving the access of dialysis services in the Kimberley region of WA and for remote communities in the Northern Territory; and development of the Chronic Kidney Disease Monitoring Centre [175].
Concluding comments
Disorders of the kidneys and urologic system pose a significant, and frequently serious, public health threat for many Indigenous people [6][7][29]. ESKD, which underlies much of the renal morbidity and mortality seen in Indigenous communities, is the clear priority for the health system, but recurring epidemics of APSGN in northern Australia, the suspected high levels of UTIs, and the unusual epidemiology of urolithiasis also require appropriate attention. Continuing high rates of ESKD, the negative social consequences that accompany treatment, and the high cost of tertiary level medical care all illustrate the immediate need for a comprehensive health care approach that addresses both the medical and socioeconomic dimensions of this major problem [30][131][159].
A range of biopsychosocial issues underlie the generally poor health status of many Indigenous people. Poverty, poor living conditions, limited access to medical care, and inadequate environmental sanitation contribute to high rates of kidney health disorders in many Indigenous communities [7][49][50]. The prevention, management and control of renal-urologic disorders will depend not only on effective, acceptable medical and surgical treatment, but, importantly, on preventive action to address the poor socioeconomic circumstances that underlie these conditions. Without adequate forward planning that considers service needs, service availability, and workforce projections, however, there will not be adequate resources to provide minimum standards of care for the growing number of Indigenous people dependant on dialysis [4][153]. A comprehensive approach that addresses both the medical and socioeconomic dimensions of these health conditions is an immediate priority.
References
- Australian Bureau of Statistics (2006) National Aboriginal and Torres Strait Islander Health Survey: Australia, 2004-05. Canberra: Australian Bureau of Statistics
- Australian Bureau of Statistics, Australian Institute of Health and Welfare (2008) The health and welfare of Australia’s Aboriginal and Torres Strait Islander Peoples 2008. Canberra: Australian Bureau of Statistics and Australian Institute of Health and Welfare
- Australian Institute of Health and Welfare (2009) An overview of chronic kidney disease in Australia, 2009. Canberra: Australian Institute of Health and Welfare
- Australia and New Zealand Dialysis and Transplant Registry (2010) The thirty third report: Australia and New Zealand Dialysis and Transplant Registry: 2010. Adelaide: Australia and New Zealand Dialysis and Transplant Registry
- Australian Institute of Health and Welfare (2011) Aboriginal and Torres Strait Islander health performance framework 2010: detailed analyses. Canberra: Australian Institute of Health and Welfare
- Preston-Thomas A, Cass A, O’Rourke P (2007) Trends in the incidence of treated end-stage kidney disease among Indigenous Australians and access to treatment. Australian and New Zealand Journal of Public Health; 31(5): 419-421
- Australian Institute of Health and Welfare (2011) Chronic kidney disease in Aboriginal and Torres Strait Islander people 2011. Canberra: Australian Institute of Health and Welfare
- Kidney Health Australia (2010) The economic impact of end-stage kidney disease in Australia – projections to 2020. Melbourne: Kidney Health Australia
- National Kidney and Urologic Diseases Information Clearinghouse (2009) The kidneys and how they work. Retrieved 2009 from http://kidney.niddk.nih.gov/kudiseases/pubs/pdf/yourkidneys.pdf
- McDonald S, Maguire GP, Hoy W (2003) Renal function and cardiovascular risk markers in a remote Australian Aboriginal community. Nephrology Dialysis Transplantation; 18(8): 1555-1561
- Hoy WE, Mathews JD, McCredie DA, Pugsley DJ, Hayhurst BG, Rees M, Kile E, Walker KA, Wang Z (1998) The multidimensional nature of renal disease: rates and associations of albuminuria in an Australian Aboriginal community. Kidney International; 54(4): 1296-1304
- Shephard MDS, Allen GG, Barratt LJ, Barbara JAJ, Paizis K, McLeod G, Brown M, Vanajek A (2003) Albuminuria in a remote South Australian Aboriginal community: results of a community-based screening program for renal disease. Rural and Remote Health; 3(1): 156 Retrieved from http://www.rrh.org.au/publishedarticles/article_print_156.pdf
- Spiers BF (2009) Antecedents of chronic kidney disease in Aboriginal offenders in New South Wales prisons: Dr Ross Ingram Memorial Essay Competition. Medical Journal of Australia; 190(10): 524-526
- Hoy WE, White AV, Dowling A, Sharma SK, Bloomfield H, Tipiloura BT, Swanson CE, Mathews JD, McCredie DA (2012) Post-streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney International; 81(2): 1026-1032
- Haysom L, Williams R, Hodson E, Lopez-Vargas P, Roy LP, Lyle D, Craig JC (2009) Risk of CKD in Australian Indigenous and non-Indigenous children: a population-based cohort study. American Journal of Kidney Diseases; 53(2): 229-237
- Haysom L, Williams R, Hodson EM, Lopez-Vargas PA, Roy LP, Lyle DM, Craig JC (2009) Natural history of chronic kidney disease in Australian Indigenous and non-Indigenous children: a 4-year population-based follow-up study. Medical Journal of Australia; 190(6): 303-306
- Haysom L, Williams R, Hodson E, Lopez-Vargas P, Roy LP, Lyle D, Craig JC (2008) Diagnostic accuracy of urine dipsticks for detecting albuminuria in Indigenous and non-Indigenous children in a community setting. Pediatric Nephrology; 24(2): 323-331
- Haysom L, Williams R, Hodson E, Roy LP, Lyle D, Craig JC (2007) Early chronic kidney disease in Aboriginal and non-Aboriginal Australian children: remoteness, socioeconomic disadvantage or race?. Kidney International; 71(8): 787-794
- The SEARCH Investigators (2010) The study of environment on Aboriginal resilience and child health (SEARCH): study protocol. BMC Public Health; (10): 287 Retrieved from http://www.biomedcentral.com/content/pdf/1471-2458-10-287.pdf
- Maple-Brown LJ, Lawton PD, Hughes JT, Sharma SK, Jones GRD, Ellis AG, Hoy W, Cass A, MacIsaac RJ, Sinha AK, Thomas MAB, Piers LS, Ward LC, Drabsch K, Panagiotopoulos S, McDermott R, Warr K, Cherian S, Brown A, Jerums G, O’Dea K (2010) Study protocol – accurate assessment of kidney function in Indigenous Australians: aims and methods of the eGFR Study. BMC Public Health; (10): 80 Retrieved from http://www.biomedcentral.com/content/pdf/1471-2458-10-80.pdf
- Hoy W, Kelly A, Jacups S, McKendry K, Baker P, MacDonald S, Wang Z, Punguatji N, Kerinauia J, Tipiloura E, Tipiloura E, Harrison C (1999) Stemming the tide: reducing cardiovascular disease and renal failure in Australian Aborigines. Australian and New Zealand Journal of Medicine; 29(3): 480-483
- Hoy W (1996) Renal disease in Australian Aboriginals: community health programs are critical to containing the renal disease epidemic [editorial]. Medical Journal of Australia; 165: 126-127
- Australian Institute of Health and Welfare (2010) Chronic kidney disease hospitalisations in Australia 2000–01 to 2007–08. Canberra: Australian Institute of Health and Welfare
- Australian Institute of Health and Welfare (2011) Australian hospital statistics 2009-10. Canberra: Australian Institute of Health and Welfare
- Australian Bureau of Statistics (2011) Causes of death, Australia, 2009. Canberra: Australian Bureau of Statistics
- Australian Institute of Health and Welfare (2008) Aboriginal and Torres Strait Islander health performance framework, 2008 report: detailed analyses. Canberra: Australian Institute of Health and Welfare
- Australian Bureau of Statistics (2010) The health and welfare of Australia’s Aboriginal and Torres Strait Islander peoples, 2010. Canberra: Australian Bureau of Statistics
- Australian Institute of Health and Welfare (2008) Australia’s health 2008: the eleventh biennial health report of the Australian Institute of Health and Welfare. Canberra: Australian Institute of Health and Welfare
- Chronic kidney disease (CKD) management in general practice (2009) Kidney Health Australia
- Kidney Health Australia (2011) Two of a KinD (kidneys in diabetes) : the burden of diabetic kidney disease and the cost effectiveness of screening people with type 2 diabetes for chronic kidney disease. Melbourne: Kidney Health Australia
- Pearle MS, Calhoun E, Curhan GC (2007) Urolithiasis. In: Litwin MS, Saigal CS, eds. Urologic diseases in America. Washington, DC: National Institutes of Health: 281-319
- Kidney stones (2009) Kidney Health Australia
- Rodriguez-Iturbe B, Musser JM (2008) The current state of poststreptococcal glomerulonephritis. Journal of the American Society of Nephrology; 19: 1855-1864
- Centre for Disease Control (2010) Northern Territory guidelines for acute post-streptococcal glomerulonephritis. Casuarina: Department of Health and Community Services, Northern Territory Government
- Dirks J, Remuzzi G, Horton S, Schieppati A, Rizvi SAH (2006) Diseases of the kidney and the urinary system. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, eds. Disease control priorities in developing countries, 2nd edition. Washington (DC): World Bank: 695-706
- Urinary tract infections (2009) Kidney Health Australia
- Carson PJ, Brewster DR (2003) Unique pattern of urinary tract calculi in Australian Aboriginal children. Journal of Paediatrics and Child Health; 39(5): 325-328
- McDonald S, Chang S, Excell L, eds (2007) Australia and New Zealand Dialysis and Transplant Registry: the thirtieth report. Adelaide: Australia and New Zealand Dialysis and Transplant Registry
- McDonald S, Excell L, Livingston B (2008) Australia and New Zealand Dialysis and Transplant Registry: the thirty first report. Adelaide: Australia and New Zealand Dialysis and Transplant Registry
- McDonald S, Excell L, Livingston B (2009) The thirty second report – Australia and New Zealand Dialysis and Transplant Registry 2009. Adelaide: Australia and New Zealand Dialysis and Transplant Registry
- Saggers S, Gray D (1991) Aboriginal health and society: the traditional and contemporary Aboriginal struggle for better health. North Sydney: Allen and Unwin
- Carson B, Dunbar T, Chenhall RD, Bailie R, eds. (2007) Social determinants of Indigenous health. Crows Nest, NSW: Allen and Unwin
- Thomson N, ed. (2003) The health of Indigenous Australians. South Melbourne: Oxford University Press
- Kamien M (1980) The Aboriginal Australian experience. In: Stanley NF, Joske RA, eds. Changing disease patterns and human behaviour. London: Academic Press: 254-270
- Moodie PM (1981) Australian Aborigines. In: Trowell H C, Burkitt DP, eds. Western diseases: their emergence and prevention. London: Edward Arnold: 154-167
- Brown A, Walsh W, Lea T, Tonkin A (2005) What becomes of the broken hearted?: coronary heart disease as a paradigm of cardiovascular disease and poor health among health among Indigenous Australians [editorial]. Heart, Lung and Circulation; 14(3): 158-162
- Wilkinson R, Marmot M (2003) Social determinants of health: the solid facts. Denmark: World Health Organization
- Thomson N, Brooks J (2003) Cardiovascular disease. In: Thomson N, ed. The health of Indigenous Australians. South Melbourne: Oxford University Press: 186-206
- Australian Health Ministers’ Advisory Council (2011) Aboriginal and Torres Strait Islander health performance framework: 2010 report. Canberra: Office for Aboriginal and Torres Strait Islander Health, Department of Health and Ageing
- Cass A, Cunningham J, Snelling P, Wang Z, Hoy W (2004) Exploring the pathways leading from disadvantage to end-stage renal disease for Indigenous Australians. Social Science & Medicine; 58(4): 767-785
- Singh GR (2009) Glomerulonephritis and managing the risks of chronic renal disease. Pediatric Clinics of North America; 56(6): 1363-1382
- Centre for Disease Control (2010) Healthy Skin Program: guidelines for community control of scabies, skin sores and crusted scabies in the Northern Territory. Darwin: Northern Territory Department of Health and Families
- White A, Wong W, Sureshkumur P, Singh G (2010) The burden of kidney disease in Indigenous children of Australia and New Zealand, epidemiology, antecedent factors and progression to chronic kidney disease. Journal of Paediatrics and Child Health; 46(9): 504-509
- Cass A, Cunningham J, Hoy W (2002) The relationship between the incidence of end-stage renal disease and markers of socioeconomic disadvantage. New South Wales Public Health Bulletin; 13(7): 147-151
- Preece C (2010) Developing a model of care to improve the health and well-being for Indigenous people receiving renal dialysis treatment. Master of Applied Science (Research) thesis, Queensland University of Technology: Brisbane
- Steering Committee for the Review of Government Service Provision (2011) Report on government services 2011: Indigenous compendium. Canberra: Productivity Commission
- Hoy WE, Kincaid-Smith P, Hughson MD, Fogo A, Sinniah R, Dowling J, Samuel T, Mott SA, Douglas-Denton RN, Bertram JF (2010) CKD in Aboriginal Australians. American Journal of Kidney Diseases; 56(5): 983–993
- Li Z, McNally L, Hilder L, Sullivan EA (2011) Australia’s mothers and babies 2009. Canberra: Australian Institute of Health and Welfare
- Hoy W (1998) Renal disease as a metaphor: towards a more integrated view of Aboriginal health. Clinical and Experimental Pharmacology and Physiology; 25(12): 1038-1042
- Spencer JL, Silva DT, Snelling P, Hoy WE (1998) An epidemic of renal failure among Australian Aboriginals. Medical Journal of Australia; 168(11): 537-541
- Thomas MAB (1998) Kidney disease in Australian Aboriginals: time for decisive action [editorial]. Medical Journal of Australia; 168(11): 532-533
- Australian Bureau of Statistics (2006) National Health Survey: summary of results, Australia, 2004-05. Canberra: Australian Bureau of Statistics
- Australia and New Zealand Dialysis and Transplant Registry (2012) ANZDATA structure. Retrieved 2012 from http://www.anzdata.org.au/v1/structure.html
- End stage renal disease notifications, by Indigenous status, age, jurisdiction and year [1995 to 2009, unpublished] (2011) Australian and New Zealand Dialysis and Transplant Registry
- Australian Bureau of Statistics (2008) Australian Demographic Statistics: June quarter 2008. Canberra: Australian Bureau of Statistics
- Australian Bureau of Statistics (2001) Australian demographic statistics: June quarter 2001. Canberra: Australian Bureau of Statistics
- Australian Bureau of Statistics (2009) Experimental estimates and projections, Aboriginal and Torres Strait Islander Australians: Projected population, Aboriginal and Torres Strait Islander Australians, Australia, states and territories, 2006-2021 series [data cube]. Retrieved 8 September 2009 from http://www.abs.gov.au/ausstats/subscriber.nsf/log?openagent&32380ds002_2006.srd&3238.0&Data%20Cubes&E79A5308D989173FCA25762A001CE1C3&0&1991%20to%202021&08.09.2009&Latest
- Australian Institute of Health and Welfare (2009) Australian hospital statistics 2007-08. Canberra: Australian Institute of Health and Welfare
- Kidney Health Australia (2006) National chronic kidney disease strategy. Melbourne: Kidney Health Australia
- Andreasyan K, Hoy WE (2010) Recent patterns in chronic disease mortality in remote living Indigenous Australians. BMC Public Health; 10: 483 Retrieved 16 August 2010 from http://dx.doi.org/10.1186/1471-2458-10-483
- Geetha D (2010) Glomerulonephritis, Poststreptococcal: overview. Retrieved from http://emedicine.medscape.com/article/240337-overview
- Scrace M, Koko K (2006) An outbreak of acute post-streptococcal glomerulonephritis in remote Far North Queensland. Australian Journal of Rural Health; 14(4): 160-163
- Mansfield K, Gunn J, Wilson N, Scott L (2011) Acute post-streptococcal glomerulonephritis and opportunistic trachoma screening in an Indigenous community in the Northern Territory, 2011. Northern Territory Disease Control Bulletin; 18(4): 8-12
- Isbel NM (2005) Glomerulonephritis – management in general practice. Australian Family Physician; 34(11): 907-9, 911-3
- Marshall C, Taylor C (2008) Acute post-streptococcal glomerulonephritis in the Northern Territory 2008. Northern Territory Disease Control Bulletin; 15(3): 1-5
- Scott L (2004) Acute post-streptococcal glomerulonephritis in a remote community. Northern Territory Disease Control Bulletin; 11(3): 7-8
- Whitehead BD, Smith HV, Nourse C (2010) Invasive group A streptococcal disease in children in Queensland. Epidemiology and Infection; 139(4): 623-628
- Holt DC, McCarthy JS, Carapetis JR (2010) Parasitic diseases of remote Indigenous communities in Australia. International Journal for Parasitology; 40(10): 1119–1126
- Binns P, Markey P, Krause V (2005) An outbreak of acute post-streptococcal glomerulonephritis in a remote Aboriginal community in 2005. Northern Territory Communicable Diseases Bulletin; 12(2): 12-15
- Andrews RM, Kearns T, Connors C, Parker C, Carville K, Currie BJ, Carapetis JR (2009) A regional initiative to reduce skin infections amongst Aboriginal children living in remote communities of the Northern Territory, Australia. PLoS Neglected Tropical Diseases; 3(11): 1-9
- McMeniman E, Holden L, Kearns T, Clucas DB, Carapetis JR, Currie BJ, Connors C, Andrews RM (2011) Skin disease in the first two years of life in Aboriginal children in East Arnhem Land. Australasian Journal of Dermatology; 52(4): 270–273
- Hudson S (2011) One disease at a time: eradicating scabies in east Arnhem Land. Policy; 27(2): 23-25
- Marshall CS, Cheng AC, Markey PG, Towers RJ, Richardson LJ, Fagan PK, Scott L, Krause VL, Currie BJ (2011) Acute post-streptococcal glomerulonephritis in the Northern Territory of Australia: a review of 16 years data and comparison with the literature. American Society of Tropical Medicine and Hygiene; 85(4): 703-710
- Clucas DB, Carville KS, Connors C, Currie B, Carapetis J, Andrews R (2008) Disease burden and health-care clinic attendances for young children in remote Aboriginal communities of northern Australia. Bulletin of the World Health Organization; 86(4): 275-281
- Steer AC, Danchin MH, Carapetis JR (2007) Group A streptococcal infections in children. Journal of Paediatrics and Child Health; 43(4): 203-213
- Gogna NK, Nossar V, Walker AC (1983) Epidemic of acute poststreptococcal glomerulonephritis in Aboriginal communities. Medical Journal of Australia; 1: 64-66
- Devanesen D, Bernard E, Stokes ML, Daby J, Withnall K, Falls G, Peach H (1987) Lessons from an outbreak of glomerulonephritis in an Aboriginal community. In: Annual Report Menzies School of Health Research. : : 86-89
- White AV, Hoy WE, McCredie DA (2001) Childhood post-streptococcal glomerulonephritis as a risk factor for chronic renal disease in later life. Medical Journal of Australia; 174: 492-496
- Hoy W, Wang Z, Vanbuynder P, Baker P, McDonald SM, Mathews J (2001) The natural history of renal disease in Australian Aboriginies: part 2, Albuminuria predicts natural death and renal failure. Kidney International; 60: 249-256
- Singh GR, Hoy WE (2004) Kidney volume, blood pressure, and albuminuria: findings in an Australian Aboriginal community. American Journal of Kidney Diseases; 43(2): 254-259
- Maple-Brown LJ, Cunningham J, Hodge AM, Weeramanthri T, Dunbar T, Lawton PD, Zimmet PZ, Chadban SJ, Polkinghorne KR, Shaw JE, O’Dea K (2011) High rates of albuminuria but not of low eGFR in urban Indigenous Australians: the DRUID Study. BMC Public Health; 11: 346 Retrieved from http://dx.doi.org/10.1186/1471-2458-11-346
- Williams S, Markey P (2005) Acute post streptococcal glomerulonephritis in a remote community. Northern Territory Communicable Diseases Bulletin; 12(2): 16-18
- Streeton C (1995) A review of the epidemiology of acute poststreptococcal glomerulonephritis and acute rheumatic fever in far North Queensland. In: Streeton C, ed. Master of Applied Epidemiology bound volume. Canberra: National Centre for Epidemiology and Population Health, Australian National University: 211-218
- McDonald MI, Towers RJ, Andrews R, Benger N, Fagan P, Currie BJ, Carapetis JR (2008) The dynamic nature of group A streptococcal epidemiology in tropical communities with high rates of rheumatic heart disease. Epidemiology and Infection; 136(4): 529-539
- McDonald MI, Towers RJ, Fagan P, Carapetis JR, Currie BJ (2007) Molecular typing of Streptococcus pyogenes from remote Aboriginal communities where rheumatic fever is common and pyoderma is the predominant streptococcal infection. Epidemiology and Infection; 135(8): 1398–1405
- Streeton C, Hanna J, Messer R, Merianos A (1995) An epidemic of acute post-streptococcal glomerulonephritis among Aboriginal children. Journal of Paediatrics and Child Health; 31(3): 245-248
- Streeton C, Hanna J (1995) Acute poststreptococcal glomerulonephritis and acute rheumatic fever in far North Queensland, 1994. Communicable Diseases Intelligence; 19(6): 137-140
- Carapetis J (1995) An outbreak of acute poststreptococcal glomerulonephritis in an Aboriginal community. Communicable Diseases Intelligence; 19(6): 140-142
- Johnston F, Carapetis J, Patel MS, Wallace T, Spillane P (1999) Evaluating the use of penicillin to control outbreaks of acute poststreptococcal glomerulonephritis. Pediatric Infectious Disease Journal; 18(4): 327-332
- McDonald M, Towers R, Fagan P, McKinnon M, Benger N, Andrews R, Currie BJ, Carapetis J (2006) Recovering streptococci from the throat, a practical alternative to direct plating in remote tropical communities. Journal of Clinical Microbiology; 44(2): 547-552
- La Vincente S, Kearns T, Connors C, Cameron S, Carapetis J, Andrews R (2009) Community management of endemic scabies in remote Aboriginal communities of northern Australia: low treatment uptake and high ongoing acquisition. PLoS Neglected Tropical Diseases; 3(5): e444 Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2680947
- Gilmore SJ (2011) Control strategies for endemic childhood scabies. PLoS ONE; 6(1): e15990 Retrieved 25 January 2011 from http://www.plosone.org/article/fetchObjectAttachment.action;jsessionid=EC322D086620F361C24C7F8EA8A8C38E.ambra02?uri=info%3Adoi%2F10.1371%2Fjournal.pone.0015990&representation=PDF
- Brown D, Edwards H, eds. (2007) Medical-surgical nursing : assessment and management of clinical problems. Marrickville, N.S.W.: Elsevier Australia
- Atkins RC (2001) How bright is their future? Post-streptococcal glomerulonephritis in Indigenous communities in Australia [editorial]. Medical Journal of Australia; 174: 489-490
- Assumpcao C (1988) Some urinary tract disease in Australian Aboriginal inpatients in 1980. Australian and New Zealand Journal of Medicine; 18(1): 17-20
- Williams R, Allam B (1992) Aboriginal renal disease awareness. Aboriginal and Islander Health Worker Journal; 16(1): 4-5
- Bookallil M, Chalmers E, Andrew B (2005) Challenges in preventing pyelonephritis in pregnant women in Indigenous communities. Rural and Remote Health; 5(3): 395 Retrieved from http://www.rrh.org.au/publishedarticles/article_print_395.pdf
- de Costa C, Child A (1996) Pregnancy outcomes in urban Aboriginal women. Medical Journal of Australia; 164(9): 523-526
- Schultz R, Read AW, Straton AY, Stanley FJ, Morich P (1991) Genitourinary tract infections in pregnancy and low birth weight: case-control study in Australian Aboriginal women. British Medical Journal; 303: 1369-1373
- Jones TW, Henderson TR (1989) Urinary calculi in children in Western Australia: 1972-86. Australian Paediatric Journal; 25(2): 93-95
- Adsett DB (1982) Urinary calculi in children of the Kimberley region of Western Australia. Australian Paediatric Journal; 18(4): 283-285
- Cassey JG, Ahmed S (1989) Urinary tract calculi in Aboriginal children. Australian Paediatric Journal; 25(6): 363-365
- Thambi Dorai C, Dewan P, Boucaut H, Ehrlich J (1994) Urolithiasis in Australian Aboriginal children. Australian and New Zealand Journal of Surgery; 64(2): 99-101
- Williams W, Nicholas J, Nungurrayi P, Napurrula C (1996) Paediatric urolithiasis in a remote Australian Aboriginal community. Journal of Paediatrics and Child Health; 32(4): 344-346
- Wisniewski ZS, Brockis JG, Ryan GD (1981) Urinary bladder stones in Aboriginal children. Australian and New Zealand Journal of Surgery; 51(3): 292-295
- Farago C (1987) Overinvestigation of Aboriginal children with urinary tract infection? [letter]. Medical Journal of Australia; 147(10): 521
- Devitt J, Devitt J, Cass A, Cunningham J, Preece C, Anderson K, Snelling P (2008) Improving access to kidney transplants (IMPAKT): a detailed account of a qualitative study investigating barriers to transplant for Australian Indigenous people with end-stage kidney disease. BMC Health Services Research; 8: 31 Retrieved 4 February 2008 from http://www.biomedcentral.com/1472-6963/8/31
- Agar JWM, Hawley CM, George CRP, Mathew TH, McDonald SP, Kerr PG (2010) Home haemodialysis in Australia — is the wheel turning full circle?. Medical Journal of Australia; 192(7): 403-406
- Northern Territory Department of Health and Families (2010) I’m OK: improving care for people with advanced chronic kidney disease. The Chronicle; 16(1): 1-4
- Prout S, Yap M (2010) Indigenous temporary mobilities and service delivery in regional service centres: a West Kimberley case study. Canberra: Centre for Aboriginal Economic Policy Research
- Carruthers D, Warr K (2004) Supporting peritoneal dialysis in remote Australia. Nephrology; 9(Supplement 4): s129-s133
- Department of Health and Community Services (2005) Renal services strategy. Darwin: Department of Health and Community Services, Northern Territory Government
- Le B, Kickett M (2009) Dislocation and dialysis in Aboriginal patients with renal failure. Aboriginal and Islander Health Worker Journal; 33(4): 10-13
- Devitt J, McMasters A (1998) Living on medicine: a cultural study of end-stage renal disease among Aboriginal people. Alice Springs: IAD Press
- Barnes J, Marfan J, Brown D (2010) The self-care dialysis program. The Chronicle; 16(1): 10-11
- Villarra A, Warr K (2004) Home haemodialysis in remote Australia. Nephrology; 9(Supplement 4): s134-s137
- Standing Committee on Aboriginal and Torres Strait Islander Health, Statistical Information Management Committee (2006) National summary of the 2003 and 2004 jurisdictional reports against the Aboriginal and Torres Strait Islander health performance indicators. Canberra: Australian Institute of Health and Welfare
- National Consumer Council of Kidney Health Australia (2010) The impact of kidney disease and what government should do about it. Melbourne: Kidney Health Australia
- David BNL, Roberts M (2008) A peritoneal-based automated wearable artificial kidney. Clinical and Experimental Nephrology; 12(3): 171-180
- Marley JV, Dent HK, Wearne M, Fitzclarence C, Nelson C, Siu K, Warr K, Atkinson D (2010) Haemodialysis outcomes of Aboriginal and Torres Strait Islander patients of remote Kimberley region origin. Medical Journal of Australia; 193(9): 516-520
- Anderson K, Devitt J, Cunningham J, Preece C, Cass A (2008) “All they said was my kidneys were dead”: Indigenous Australian patients’ understanding of their chronic kidney disease. Medical Journal of Australia; 189(9): 499-503
- Lowe M, Kerridge IH, Mitchel KR (1995) ‘These sorts of people don’t do very well’: race and allocation of health care resources. Journal of Medical Ethics; 21(6): 356–360
- MacMahon J, Stewart S (2010) Your future: advanced care planning for renal clients. The Chronicle; 16(1): 17
- Tonelli M, Wiebe N, Knoll G, Bello A, Browne S, Jadhavb D, Klarenbacha S, Gillc J (2011) Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. American Journal of Transplantation; 11(10): 2093-2109
- Mathew T, Faull R, Snelling P (2005) The shortage of kidneys for transplantation in Australia: editorial. Medical Journal of Australia; 182(5): 204-205
- McDonald SP, Russ GR (2002) Survival of recipients of cadaveric kidney transplants compared with those receiving dialysis treatment in Australia and New Zealand, 1991–2001. Nephrology Dialysis Transplantation; 17(12): 2212-2219
- Cass A, Cunningham C, Snelling P, Wang Z, Hoy W (2003) Renal transplantation for Indigenous Australians: identifying the barriers to equitable access. Ethnicity & Health; 8(2): 111-119
- Cass A, Cunningham J, Wang Z, Hoy W (2001) Regional variation in the incidence of end-stage renal disease in Indigenous Australians. Medical Journal of Australia; 175: 24-27
- How the waiting lists operate: organ donation (2009) Australian Organ and Tissue Donation and Transplantation Authority
- Johnston R (2010) Chronic kidney disease: a coordinated approach. The Chronicle; 16(1): 5
- Amega B, Bowen E (2010) Together – no more them and us!. The Chronicle; 16(1): 6-7
- Race K, Nash F (2010) NT Australian Better Health Initiative (ABHI) project: primary care integration. The Chronicle; 16(1): 8-9
- Stewart S (2010) Palliative care for renal clients living in a remote setting. The Chronicle; 16(1): 12-13
- Program of Experience in the Palliative Approach learning guide for Aboriginal and Torres Strait Islander Health Workers (2011) Program of Experience in the Palliative Approach
- Stewart S (2010) “Palliative care for sick kidneys” education resource. The Chronicle; 16(1): 18
- Murphy S (2010) A journey home to country. The Chronicle; 16(1): 16
- Gorham G (2010) Renal Indigenous resources project. The Chronicle; 16(1): 19
- Gorham G (2003) Prevention and treatment options for renal disease in the Northern Territory (with particular reference to the Barkly region). Casuarina: Cooperative Research Centre for Aboriginal and Tropical Health
- Couzos S, Thomas M, Cass A (2008) Chronic kidney disease. In: Couzos S, Murray R, eds. Aboriginal primary health care: an evidence-based approach. 3rd ed. South Melbourne: Oxford University Press: 575-621
- Menzies School of Health Research (2000) Menzies School of Health Research 1999-2000 Annual Report. Darwin: Menzies School of Health Research
- Hoy WE, Baker PR, Kelly AM, Wang Z (2000) Reducing premature death and renal failure in Australian Aboriginals: a community-based cardiovascular and renal protective program. Medical Journal of Australia; 172: 473-478
- Menzies School of Health Research (1999) 1998-1999 Annual Report. Darwin: Menzies School of Health Research
- Hoy W, Vanbuynder P, Mathews J, Pugsley D, Wang Z (2001) Renal disease and the environment: lessons from Aboriginal Australia. Nephrology; 6: 19-24
- McDonald SP, Russ GR (2003) Current incidence, treatment patterns and outcome of end-stage renal disease among Indigenous groups in Australia and New Zealand. Nephrology; 8(1): 42-48
- Stewart JH, McCredie MRE, McDonald SP (2004) The incidence of treated end-stage renal disease in New Zealand Maori and Pacific Island people and in Indigenous Australians. Nephrology Dialysis Transplantation; 19(3): 678-685
- Stewart JH, McCredie MRE, McDonald SP (2004) Incidence of end-stage renal disease in overseas-born, compared with Australian-born, non-Indigenous Australians. Nephrology; 9(4): 247-252
- Cunningham J (2007) Making an IMPAKT: a national study aimed at improving access to optimal treatment for people with end-stage kidney disease. The Chronicle; 10(2): 17-18
- Anderson K, Cass A, Cunningham J, Snelling P, Devitt J, Preece C (2007) The use of psychosocial criteria in Australian patient selection guidelines for kidney transplantation. Social Science & Medicine; 64(10): 2107-2114
- Cunningham J, Cass A, Arnold PC (2005) Bridging the treatment gap for Indigenous Australians: demands for efficiency should not be met at the expense of equity [editorial]. Medical Journal of Australia; 182(10): 505-506
- NSW Health (2010) Kidney Health Check: promoting the early detection and management of chronic kidney disease. North Sydney: NSW Health
- Chadban S, Howell M, Twigg S, Thomas M, Jerums G, Alan C, Campbell D, Nicholls K, Tong A, Mangos G, Stack A, McIsaac R, Girgis S, Colagiuri R, Colagiuri S, Craig J (2009) National evidence based guideline for diagnosis, prevention and management of chronic kidney disease in type 2 diabetes. Canberra: National Health and Medical Research Council
- Hoy WE, Wang Z, Baker PRA, Kelly AM (2003) Secondary prevention of renal and cardiovascular disease: results of a renal and cardiovascular treatment program in an Australian Aboriginal community. Journal of the American Society of Nephrology; 14(supplement): 178-185
- National Health Priority Action Council (2006) National chronic disease strategy. Canberra: Australian Government Department of Health and Ageing
- Howard K, Salkeld G, White S, Chadban S, Craig J, McDonald S, Perkovic V, Cass A (2006) The cost-effectiveness of early detection and intervention to prevent the progression of chronic kidney disease in Australia. Melbourne: Kidney Health Australia
- Thomas MC (2007) Early detection of patients with kidney disease. Nephrology; 12(s1): S37
- Australian Institute of Health and Welfare (2009) Outline of the National Centre for Monitoring Chronic Kidney Disease. Canberra: Australian Institute of Health and Welfare
- The George Institute for Global Health (2011) Australian Department of Health and Ageing Central Australia Renal Study. Canberra: Australian Department of Health and Ageing
- Cass A, Chadban S, Craig J, Howard K, McDonald S, Salkeld G, White S (2006) The economic impact of end-stage kidney disease in Australia. Melbourne: Kidney Health Australia
- Cass A, Devitt J, Preece C, Cunningham J, Anderson K, Snelling P, Eris J, Ayanian J (2004) Barriers to access by Indigenous Australians to kidney transplantation: the IMPAKT study. Nephrology; 9(Supplement 4): s144-s146
- Coulehan K, Brown I, Christie M, Gorham G, Lowell A, Marrnanyin, Patel B (2005) Sharing the true stories: evaluating strategies to improve communication between health staff and Aboriginal patients: stage 2 report: September 2002 to August 2005. Darwin: Cooperative Research Centre for Aboriginal Health
- Human Rights and Equal Opportunity Commission (2008) Close the gap: Indigenous health equality summit, statement of intent. Canberra: Human Rights and Equal Opportunity Commission
- Council of Australian Governments (2009) National Indigenous reform agreement (closing the gap). Canberra: Council of Australian Governments
- COAG Reform Council (2011) National Indigenous Reform Agreement: performance report for 2009–10. Sydney: COAG Reform Council
- Council of Australian Governments (2009) National framework for protecting Australia’s children 2009–2020: protecting children is everyone’s business. Canberra: Council of Australian Governments
- Roxon N, Mclucas J (2009) Kidney disease – Australia’s ‘silent killer’. Retrieved 26 May 2009 from http://www.health.gov.au/internet/ministers/publishing.nsf/Content/1069A4C8CDC02D6ACA2575C2000AC3D0/$File/nr073.pdf
Acknowledgements
Special thanks are extended to:
- the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA) for the provision of the notification data on end-stage kidney disease (ESKD). (Interpretation and reporting of these data are the responsibility of the authors, however, and in no way should be seen as an official interpretation of ANZDATA.)
- Associate Professor Stephen McDonald for his expert guidance in the preparation of the review
- other staff of the Australian Indigenous HealthInfoNet, particularly to Leah Levitan, for their assistance, support and encouragement in the preparation of this review
- the Office for Aboriginal and Torres Strait Islander Health (OATSIH) within the Australian Department of Health and Ageing for their ongoing support of the work of the HealthInfoNet.
Endnotes
- The NATSIHS collected information from people living in private dwellings and did not include people in health care facilities, so may underestimate the prevalence of kidney disease in the Indigenous population.
- These data refer to episodes of admitted care, meaning the same patient can potentially have multiple hospitalisations within the same period. Consequently, data represents health service usage by those with CKD rather than representing the number or proportion of people in Australia with CKD admitted to hospital.
- Indigenous identification in deaths data is considered of sufficient quality for reporting only for NSW, Qld, WA, SA and the NT.
- The Index of Relative Advantage and Disadvantage is one of the ABS’s Socio-Economic Indexes For Areas (SEIFA) 2006, generated using a combination of 2006 Census data (i.e. income, education and dwelling size). Composite scores are indicative of the collective socioeconomic status of the people living in an area, with reference to the situation and standards applying to the wider community at a given point in time [68].
- Data do not represent the number or proportion of people in Australia with CKD admitted to hospital but rather health service usage by those with CKD.
- Due to data quality issues relating to Indigenous identification, hospital data by Indigenous status was restricted to hospitals in NSW, Vic, Qld, WA SA and public hospitals in the NT and hospitalisations where Indigenous status was missing or unknown were amalgamated with those identifying as non-Indigenous or ‘other’ Australians.
- The National Consumer Council of Kidney Health Australia advocates for the interests of kidney patients and carers throughout Australia. It comprises kidney patients and carers working together to improve the quality of life for people affected by kidney disease [128].
Stumpers S1, Thomson N1
Review of kidney disease among Indigenous people. Australian Indigenous HealthBulletin 13(2). Retrieved [access date] from http://healthbulletin.org.au/articles/review-of-kidney-disease-among-indigenous-people
1Australian Indigenous HealthInfoNet
Sasha Stumpers, the principal author of this review, passed away unexpectedly on 23 September 2012. A well-liked and highly esteemed member of the HealthInfoNet team, Sasha is deeply missed.
View PDF version (PDF – 538 KB)
Introduction
Kidney disease is a significant health problem for all Australians, but severe kidney disease is more common among Indigenous people than among non-Indigenous people [1][2]. In particular, the prevalence of chronic kidney disease (CKD) (Box 1) [2][3][4][5][6] and the overall levels of end-stage kidney disease (ESKD) among Aboriginal and Torres Strait Islander peoples are consistently reported as significantly higher than among other Australians [4][7].
Box 1. Chronic kidney disease and end-stage kidney disease
Chronic kidney disease (CKD) is defined as kidney damage or reduced kidney function that lasts for three months or more [7][8][9]. Diabetes (diabetic nephropathy) and high blood pressure are the most common causes of CKD; other causes include glomerular disease, inherited disorders (such as polycystic kidney disease), and hypertensive renal disease.
The most severe form of chronic kidney disease, known as end-stage kidney disease (ESKD; also known as end-stage renal disease (ESRD)), occurs when kidney function has decreased to the point where kidney replacement therapy (KRT) is necessary to avoid death. KRT involves either dialysis (mechanical filtering of the blood to help maintain functions normally performed by the kidneys) or transplantation (implantation of a kidney from either a living or recently deceased donor). (See also Box 8.)
CKD is expensive to treat and has a marked impact on the quality of life of those who suffer from the disease as well as those who care for them [7][8]
Information on CKD among Indigenous Australians is available from self-reported survey data, as well as from community-based studies and screening programs [10][11][12][13][14][15][16][17][18][19][20] but the main focus in the literature has been on ESKD [4][7]. The incidence of ESKD is especially high for Indigenous people living in remote and very remote areas of Australia (Derived from [5]) with rates of ESKD highest in northern Australian Indigenous communities [4][6][21][22].
People with CKD require extensive hospital services, particularly those patients with ESKD who require kidney replacement therapy (KRT) to survive. As such, CKD is a significant cause of hospitalisation for the Indigenous Australian population [23]; this is particularly the case for dialysis, the form of KRT on which far greater proportions of Indigenous people with ESKD than their non-Indigenous counterparts rely [4]. In 2009-10, care involving dialysis was the most common reason for the hospitalisation of Indigenous Australians: they were hospitalised at 11 times the rate of other Australians [24].
Indigenous people have substantially higher death rates than other Australians from most causes [25][26] and diseases of the kidney and urinary system are one of the top ten leading causes of death overall, for all Australians, including Indigenous people [25]. Indigenous people are more likely to die from kidney disease that non-Indigenous people, with the death rate ratios being particularly high after the age of 25 years for both Indigenous males and females compared with rates for non-Indigenous Australians [7][27].
About this review
The purpose of this review is to provide an overview of the burden of kidney disease among Indigenous Australians. After summarising kidney disorders and the context of kidney disease among the general Australian population, attention is directed to the factors contributing to kidney disease among Indigenous people. The extent of CKD and ESKD among Indigenous people in Australia is then addressed by outlining the overall incidence and prevalence, and associated hospitalisation and mortality. The review also outlines the extent of other urologic conditions and disorders of the urinary tract among Indigenous people, including acute post-streptococcal glomerulonephritis (APSGN), urinary tract infections (UTIs) and urolithiasis. The factors contributing to kidney health problems, particularly CKD and ESKD, are considered in the context of the management and treatment of these conditions. The review concludes by outlining the current policies and strategies employed to address kidney health in Australia, with particular focus on polices and strategies employed to address the kidney health of Indigenous Australians.
Kidney disorders
‘Kidney disease’, ‘renal disease’ and ‘renal disorder’ are collective terms that refer to a variety of different renal and urologic disease processes involving damage to the working units of the kidneys and also includes conditions affecting the function of the body’s urinary system, involving the ureters, bladder and urethra [9]. The loss of kidney function (Box 2) can have a serious impact on the body, causing damage to other organs and bodily processes and, as a result, a number of complications and co-morbidities often occur, including: heart disease; infections; as well as problems with bones and muscles [7][28].
Box 2. The kidneys and their function
The two kidneys, parts of the urinary tract system, regulate the mineral composition, water content and acidity of the body [9]. They are also involved in the excretion of metabolic waste products and chemicals, are responsible for the production of certain hormones and vitamins, and also have a key role in blood pressure regulation. Removal of wastes occurs in tiny units inside the kidney known as nephrons; inside each nephron is a glomerulus which acts as a sieve-like filtering unit keeping proteins and cells in the bloodstream while allowing wastes to pass through. These wastes and any extra water become urine, which passes through tubes called the ureters into the bladder where it is stored until released during urination. Damage to the working units of the kidneys results in a reduction in the filtering capacity of one or both kidneys [9][29]. Progressive or repeated damage ultimately leads to a point where the kidneys are unable to maintain life, leading to death from ESKD unless a form of renal replacement therapy (dialysis or transplantation) is used.
Due to the commonality of the risks factors for other chronic diseases (such as diabetes, hypertension and other cardiovascular disease), kidney disease often occurs with concurrent or co-existing conditions [7][29][30]. Co-morbidities can appear or be worsened because of CKD, or they can arise independently. They contribute greatly to the development of CKD and ESKD [10][12][29]. Treatment required for those who have reached the stage of ESKD involves KRT (also known as renal replacement therapy (RRT)), involving transplantation of the kidney or dialysis [9][23].
Other important kidney and urologic disorders include APSGN, UTIs, and urolithiasis [31][32][33][34]. The unusual epidemiology of urolithiasis within Indigenous communities and the potential for recurrent or persistent UTIs to trigger more serious kidney damage when coupled with conditions such as diabetes [35], have earmarked these urologic conditions as important health issues also [35][36][37].
Context of chronic kidney disease in Australia
In Australia, the three major causes for ESKD since 2005 have been diabetes, glomerulonephritis (diseases where there is inflammation of the glomeruli) and renovascular disease/hypertension [4][38][39][40]. Abnormal lipid metabolism, proteinuria, sleep apnoea, mineral and bone disorder, anaemia, dietary protein restriction, poor nutrition, uraemia, acidosis, hyperkalaemia, restless legs, and depression are some of the other associated problems with impairment in kidney function [29]. The risk of such complications progressively increases as kidney function declines [28].
Factors contributing to kidney disease among Indigenous Australians
Factors contributing to kidney disease among Indigenous people are complex. They reflect a combination of broad historical, social, cultural, and economic factors, as well as the more commonly described proximal biomedical risk factors. The importance of historical, psychosocial and socioeconomic aspects is recognised, but it is beyond the scope of this review to address these factors in any great detail. It is also beyond the scope of the review to address comorbidity with diseases such as diabetes, which is recognised as an important contributing risk factor. As a result, the main risk factors addressed in this review are behavioural and biomedical risk factors.
Historical and socioeconomic context
The current health disadvantages experienced by Indigenous people can be linked to social disadvantages and should be viewed within a broad historical context [41][42]. The kidney health of Indigenous people has been affected by changes to their once active lifestyles and roles, brought about by displacement and colonisation by European settlers [43][44][45][46]. The reduction in activity levels of Indigenous people over time, coupled with poor nutrition resulting from displacement by Europeans, has become embedded in the social foundations and reflected in the social determinants of health [47]. This has played a part in the development of kidney disease and other chronic conditions such as diabetes over time, particularly in the later part of the 20th Century [45].
In addition to the modifiable risk factors (i.e. diet, obesity) for kidney health and other chronic conditions impacted by changes to the roles of Indigenous people [43][45][48], inadequate social and economic circumstances also underlie the generally poor health status of many Indigenous people and contribute to the high rates of kidney and urinary tract disorders in many Indigenous communities. Factors relating to the health-care system and government policies include limited access to primary and other medical care [49][50]. Sub-standard living conditions, inadequate environmental sanitation and poverty also play major contributing roles [7][50]. In Indigenous communities, prevention, control and management of kidney disease and associated disorders will depend not only on effective, acceptable treatment, but also on preventive action to address the poor socioeconomic conditions that underlie these conditions [51][52][53][54][55].
Behavioural and biomedical risk factors
The factors contributing to kidney disease among Indigenous people are generally attributed to various combinations of multiple risk factors including: repeated infections, high blood pressure, obesity [7][56], low birth weight [57][58], infant malnutrition [59][60][61], engagement in high risk behaviours that can adversely affect health (e.g. poor diet, low activity levels, alcohol and tobacco use) [1][2][50] and diabetes [2][4]. These conditions are quite common among Indigenous people, particularly Indigenous women, and contribute to high rates of kidney disease [2][23][56]. Given the modifiable nature of a majority of these factors, efforts to minimise these risks can help to reduce the prevalence of kidney disease and its associated mortality.
Extent of CKD and end-stage kidney disease (ESKD) among Indigenous people
Incidence and prevalence
As noted previously, the level of CKD is higher among Aboriginal and Torres Strait Islander people than among other Australians [2][3][4][5][6]. With no large-scale Indigenous biomedical survey available, it is necessary to rely on self-reported survey data as well as information from community-based reports and screening programs to estimate the prevalence of CKD among Indigenous people [10][11][12][13][14][15][16][17][18][19][20][28]. The most recent self-reported information available is from the 2004-05 National Aboriginal and Torres Strait Islander Health Survey (NATSIHS) with comparable data for non-Indigenous people available from the 2004-05 National Health Survey (NHS) [1][27][28][62].
In 2004-2005, kidney disease was reported by 2% of Indigenous people overall1 [1][2]. After age-adjustment, kidney disease was ten times more common among Indigenous people than among non-Indigenous people [1][2][27]. The prevalence of kidney disease for Indigenous people increased with age, from less than 1% in the 0-14 age group to 7% for Indigenous people aged 55 years and over (see Figure 1) [1]. Except for the 0-14 years age group, kidney disease was much more common among Indigenous people than among non-Indigenous people across all age groups. The level of kidney disease reported in the 2004-2005 NATSIHS was nearly twice that reported in 2001 [1][2][27].
Figure 1. Prevalence of kidney disease, by Indigenous status and age, Australia 2004-2005
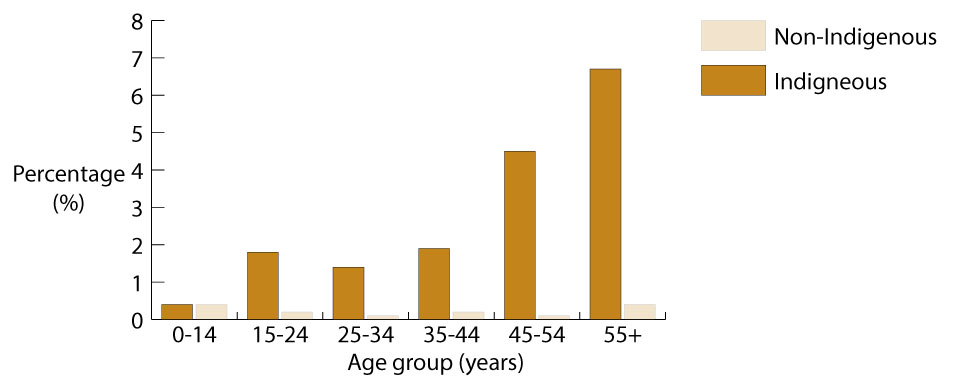
Source: [1].
Detailed information about CKD is only available from periodic health surveys, but progression to ESKD is reported routinely to the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA), which collates the data and produces annual surveillance reports [4][7][63]. These reports reveal that the overall incidence of ESKD is consistently significantly higher among Indigenous people than among non-Indigenous people [3][4].
A total of 675 Indigenous people were newly identified with ESKD between 2007 and 2009 – the age-standardised notification rate of 960 per 1,000,000 population for Indigenous people was almost 10 times the rate of 97 per 1,000,000 for non-Indigenous people (Table 1) (Derived from [64][65][66][67]). Incidence rates of ESKD were higher for Indigenous people than for non-Indigenous people in all states and territories, with the highest rates recorded for Indigenous people living in the NT (1,594 per 1,000,000), WA (1,194 per 1,000,000), and SA (876 per 1,000,000).
Table 1: Incidence and age-standardised incidence rates for end-stage kidney disease, by Indigenous status, and Indigenous:non-Indigenous rate ratios, selected jurisdictions, Australia, 2007-2009
| Jurisdiction | Indigenous | Non-Indigenous | Rate ratio | ||
|---|---|---|---|---|---|
| Number | Rate | Number | Rate | ||
| Source: Derived from [64][65][66][67]. | |||||
Notes:
|
|||||
| NSW | 80 | 283 | 2,200 | 98 | 2.9 |
| Vic | 19 | 415 | 1,601 | 101 | 4.1 |
| Qld | 167 | 637 | 1,318 | 106 | 6.0 |
| WA | 156 | 1,194 | 605 | 97 | 12.4 |
| SA | 44 | 876 | 503 | 107 | 8.2 |
| NT | 201 | 1,594 | 36 | 89 | 18.0 |
| Australia | 675 | 960 | 6,574 | 97 | 9.9 |
More than three-fifths (63%) of Indigenous people newly registered with ANZDATA in the period 2007-2009 were aged less than 55 years, compared with less than one-third (30%) of non-Indigenous people registered (Derived from [64][65][66][67]) (Table 2). Age-specific notification rates were higher for Indigenous people than for non-Indigenous people across all age groups except those aged 0-14 years. Rate ratios were particularly high for people aged 35-44 years (12.6) and 45-54 years (14.3).
Table 2: Incidence and age-standardised incidence rates of end-stage kidney disease, by Indigenous status and age group, and Indigenous:non-Indigenous rate ratios, Australia, 2007-2009
| Age (years) | Indigenous | Non-Indigenous | Rate ratio | ||
|---|---|---|---|---|---|
| Number | Rate | Number | Rate | ||
| Source: Derived from [64][65][66][67]. | |||||
Notes:
|
|||||
| 0-14 | 1 | 2 | 83 | 7 | 0.2 |
| 15-24 | 12 | 37 | 155 | 18 | 2.1 |
| 25-34 | 48 | 214 | 313 | 36 | 5.9 |
| 35-44 | 143 | 703 | 507 | 56 | 12.6 |
| 45-54 | 222 | 1,528 | 932 | 107 | 14.3 |
| 55-64 | 183 | 2,239 | 1,360 | 190 | 11.8 |
| 65-74 | 59 | 1,670 | 1,667 | 375 | 4.5 |
| 75+ | 7 | 438 | 1,557 | 390 | 1.1 |
| All ages | 675 | 960 | 6,574 | 97 | 9.9 |
The high rates of ESKD are a major public health problem for Indigenous people, particularly those living in remote parts of the country. In 2006-2008, the incidence of ESKD varied greatly with remoteness [5]. The incidence of ESKD for Indigenous people was especially high in remote and very remote areas of Australia with rates almost 18 times and 20 times those of their non-Indigenous counterparts (Table 3).
Table 3: Standardised incidence rates of end-stage kidney disease, by Indigenous status, rate ratios, and remoteness, 2006-2008
| Remoteness | Indigenous (No. per 100,000) | Non-Indigenous (No. per 100,000) | Rate ratio |
|---|---|---|---|
| Source: Derived from [5]. | |||
Notes:
|
|||
| Major cities | 40 | 11 | 3.6 |
| Inner regional | 40 | 9 | 4.3 |
| Outer regional | 98 | 9 | 11.3 |
| Remote | 149 | 8 | 17.7 |
| Very remote | 168 | 9 | 19.9 |
| Australia | 80 | 10 | 8.0 |
The full extent of ESKD among Indigenous people is reflected by the far greater proportion of Indigenous people reliant on dialysis for KRT compared with their non-Indigenous counterparts [4]. As at 31 December 2008, of all Indigenous ESKD patients registered with ANZDATA, 88% relied on dialysis compared with 55% of non-Indigenous Australians [5]. Only 12% of Indigenous people had received a kidney transplant, compared with 45% of their non-Indigenous counterparts.
Hospitalisation
A recent report on hospitalisation confirms that Indigenous people are admitted to hospital much more frequently for CKD and ESKD than are non-Indigenous Australians [23]. In 2009-10, care involving dialysis was the most common reason for the hospitalisation (Box 3) of Indigenous people living in NSW, Vic, Qld, WA, SA and the NT, with Indigenous people hospitalised 11 times more often than were their non-Indigenous counterparts [24]. In 2007-08, Indigenous females had the highest rate of dialysis hospitalisations, almost 15 times that of other females, and Indigenous males were 8 times more likely to be hospitalised for dialysis than were other males [23]. For the same period, the hospitalisation rate of Indigenous people for the procedure of dialysis was more than 12 times that of non-Indigenous people [68].
Box 3. Information about hospitalisation
Hospitalisation data are not a good reflection of the level of CKD in the community, but can give some indication of the impact of the disease as well as provide information on who is accessing services. Dialysis treatment is the most common reason for hospitalisation in Australia, with patients on dialysis needing to attend a hospital or satellite centre (attached to a hospital) three times a week for treatment [23][49]. A person who has frequent admissions for the same disease (such as dialysis) is counted multiple times, so it is important to separate hospitalisation rates for dialysis from rates for other conditions [49].
In 2007-08, the most recent period for which this information is available, Indigenous people living in NSW, Vic, Qld, WA, SA and the NT were five times more likely than their non-Indigenous counterparts to be hospitalised for principal or additional CKD diagnoses other than care for dialysis [23]. Hospitalisation rates for CKD as both principal diagnosis and for additional diagnoses were between five and seven times higher for Indigenous females than for other females2.
Mortality
In 2004-2008, the age-standardised death rate for kidney disease among Indigenous people living in NSW, Qld, SA WA and the NT was 5.1 times the rate for their non-Indigenous counterparts3 [56]. The death rates for kidney disease for Indigenous people living in NSW, Qld, WA, SA and the NT, increased by 102% over the eight-year period 2001-2008, compared with a 23% increase for their non-Indigenous counterparts [5].
Earlier data from 2001-2005 reveal that death rates involving CKD were especially high after the age of 25 years for both Indigenous males and females compared with the rates for their non-Indigenous counterparts [27]. In the 45-54 years age group, Indigenous males were 31 times more likely than non-Indigenous males to die from CKD. For the same age group, Indigenous females were 51 times more likely to die from CKD than were non-Indigenous females.
Deaths involving CKD can occur in the context of other chronic conditions, so published death rates for CKD underestimate its contribution to mortality [7][69]. For example, a common underlying cause of death for Indigenous Australians living in NSW, Qld, WA, SA and the NT in 2003-2007 where CKD was an associated cause (Box 4) was diabetes (28%), but for non-Indigenous Australians this was the equal least common underlying cause of death (8%) [7].
Box 4. Underlying and associated causes of death
Cause of death coding provides for classification according to whether a condition was an underlying or associated cause of death [3][7]. This means that CKD can be coded as the underlying cause (i.e. either led directly to an individual’s death) or coded as an associated cause (i.e. contributed to the death but was not related to the condition causing it) [7].
Table 4: Underlying cause of death where CKD was an associated cause, by underlying causes and Indigenous status, NSW, Qld, WA, SA and NT, 2003-2007
| Underlying cause of death (ICD-10 codes) | Indigenous | Non-Indigenous | ||
|---|---|---|---|---|
| Number | Per cent | Number | Per cent | |
| Source: [7]. | ||||
Notes:
|
||||
| Cardiovascular diseases (100-199) | 326 | 30 | 13,816 | 44 |
| Diabetes (E10-E14) | 303 | 28 | 2,432 | 8 |
| Neoplasms (C00-D48) | 107 | 10 | 6,062 | 19 |
| Respiratory diseases (J00-J99) | 88 | 8 | 2,636 | 8 |
| Other (balance) | 263 | 24 | 6,634 | 21 |
| Total | 1,087 | 100 | 31,580 | 100 |
An analysis of deaths from kidney disease for the period 2002-2005 found that the age- standardised rate for Indigenous people living in remote areas was higher than the rates for those living in outer regional and very remote areas (7.9 per 1,000 population compared with 4.6 and 4.8 respectively) [70]. The higher rate for remote areas than for very remote areas is contrary to expectations. The authors raised the possibility that this could be at least partly due to a better social environment, increased physical activity, healthier diet and lower consumption of alcohol and other drugs in very remote areas compared with remote areas. An alternative explanation is that the difference in death rates could be due to selection bias; that is, people from very remote areas tend to move to population centres for better access to health services for themselves or sick family members.
Other kidney health disorders
Glomerulonephritis
Like the broad terms ‘kidney disease’ and ‘renal disorder’, ‘glomerulonephritis’ is a collective term that refers to a number of pathologies that cause inflammation of the glomerulus and subsequent damage to the filtration process of the kidney [35][43]. Of the various conditions covered by the term, acute post-streptococcal glomerulonephritis (APSGN; see Box 5) is the most important contributor to high rates of chronic kidney disease and continues to pose a significant public health problem in developing countries and among Indigenous populations of the developed world [33][35].
Box 5. Acute post-streptococcal glomerulonephritis (APSGN)
APSGN is a potentially serious, non-suppurative condition that occurs two to three weeks after skin or throat infection with some strains of group A streptococcus (GAS) bacteria [34][71]. Occasionally, infections with groups C or G streptococcus may also cause APSGN [34]. ASPGN can cause high blood pressure, haematuria with reduced serum complement levels, albuminuria and oedema, and is often complicated with acute kidney failure [34][72]. The usual period between infection and development of APSGN is 1-4 weeks with an average of 10 days, but this may be longer (3-6 weeks) after streptococcal skin infections or shorter (1-2 weeks) after streptococcal throat infections [73][74].
Extent of acute post-streptococcal glomerulonephritis (APSGN) among Indigenous people
Incidence and prevalence
APSGN has been uncommon among affluent populations of developed countries since the mid-1900s due mainly to the improvements in living conditions and later because of the availability of antibiotic therapy and improved access to health care [33]. APSGN is relatively uncommon in the general Australian population, but poses a significant public health problem in tropical Australia where pyoderma, skin infections and scabies are endemic [34][72][75][76][77][78][79][80] with up to 50% of the children infested with scabies [52][81][82]. The incidence of APSGN in the NT is among the highest in the world with a rate in Indigenous children under 15 years of 94 per 100,000 population reported for the period 1991 to 2008 [34][83].
Most cases of APSGN follow skin infections rather than throat infections because skin infections are the most pervasive problem [34][72]; children aged between 12 months and 17 years are predominantly affected [34]. Secondary infection of scabies with GAS, which occurs in 50-70% of cases, is the principal cause of APSGN [84]. APSGN outbreaks continue to occur in northern Australia with a yearly peak between April and June of sporadic cases and small clusters; larger and more widespread outbreaks occur around every five years across the Top End [34][75].
Results of studies on the contribution of post-streptococcal glomerulonephritis (PSGN) to chronic kidney disease vary, but recent research [14][53][84][85], and previous research undertaken in isolated Indigenous communities experiencing outbreaks of APSGN in 1980 [86] and 1987 [87] suggest that a history of APSGN in childhood is a risk factor for subsequent kidney dysfunction [14][83][88]. Findings from an early study, which used the albumin to creatinine ratio (a sensitive early marker of kidney damage) as its main outcome measure, suggest that about one-quarter of cases of overt albuminuria may be attributable to APSGN in childhood [88]. Albuminuria has since been shown to mark early chronic kidney disease in Aboriginal and Torres Strait Islander people [11][12][14][89][90][91]. Detailed consideration of the early markers of kidney disease is beyond the scope of this review, but current available data suggest that albuminuria may be a better prognostic marker of kidney disease than a low estimated glomerular filtration rate, which may be inaccurate in the Indigenous population based on comparisons made between an urban Indigenous population and those from the general Australian adult population [91].
APSGN outbreaks in Indigenous communities
Outbreak investigations and surveillance studies provide some indication of the extent of APSGN within Indigenous communities [72][73][75][76][79][83][92] but methodological difficulties surrounding the identification of cases and the estimation of rates suggest that the true incidence of APSGN is likely to be higher than these estimates [83][93]. Identification issues include: lack of a standardised case definition; limitations and logistical difficulties associated with diagnosing the condition in isolated communities [33][93]; and the high proportion of sub-clinical cases of disease [33][34].
In the last decade, Indigenous children with skin infections during APSGN outbreaks were five times more at risk to develop ASPGN than were their non-Indigenous counterparts [80]. In 2008, an outbreak was recorded in the NT where a 5 year old child first presented with GAS and subsequently APSGN. In the following two days, two more cases were found. A complete screening of school-aged children (under 15 years) revealed that 36% of the screened children presented with skin sores and 12.5% presented with scabies, but no other case of APSGN was found [75]. In 2007, prospective surveillance investigating the epidemiology of GAS in three remote Aboriginal communities in the NT found that inadequate health awareness, crowded household conditions and population mobility were important driving forces behind GAS dissemination, with household acquisition commonly transmitted via 5-9 year olds [94][95]. Responses to APSGN outbreaks have seen improvements in screening, but Indigenous communities in the NT continue to experience epidemics of APSGN around every 5-7 years with the last known outbreak occurring in 2008 [73].
Numerous outbreaks in the NT have received rigorous investigation and sound documentation since the early 1980s [73][75][76][79][80][83][86][87][92][95][96][97][98], but, until more recently, epidemics have been less frequently documented in other areas of northern Australia. Prior to 1993, not a single epidemic had been reported in far north Qld for almost 20 years, but 58 cases (10% of all children screened) were diagnosed among Indigenous children from three remote communities in that year [96] and in 2006, an outbreak of ASPGN was recorded in the Lockhart River community in far north Qld [72]. From over 87 children screened, 46% were infected by scabies. In a four month period, 11 cases of ASPGN were confirmed. More recently, a retrospective review of the clinical records of all cases of invasive group A streptococcal (iGAS) disease notified to Qld health authorities during the 5-year period 2004-2009 revealed that there was marked underreporting in Qld [77]. Even with reports across different districts differing markedly and a large number of isolates not reported, rates of invasive disease were higher in the Indigenous population than in the non-Indigenous population. As revealed by previous research, rates of iGAS were especially high in infants, with the incidence 10 times higher among Indigenous infants than among their non-Indigenous counterparts (123 per 100,000 compared with 12 per 100,000).
Mortality and morbidity
The clinical course of APSGN has been described usually as mild, with complete recovery within days or weeks [34][86][88][99]. The long-term outlook has generally been regarded as excellent [88], but, despite the apparently benign clinical profile, APSGN has also been associated with significant morbidity, hospitalisation and occasional mortality [93][97]. Recent information on mortality and morbidity associated with APSGN are not available, but it is known that APSGN may occasionally cause acute kidney failure and that hypertension is the most frequent major complication [34].
Earlier research investigating the morbidity associated with APSGN in far north Qld reported that of 100 identified cases (of which 96 were Indigenous), 72 were admitted to hospital where they remained an average of 8.5 days (range: 3-30 days) [93][97]. Major complications included severe hypertension (40 per cent of all cases), acute kidney failure (18 per cent of all cases), fluid overload/pulmonary oedema (12 per cent of all cases) and bacteraemia (6 per cent of all cases). One child died, another developed pneumococcal pericarditis and a purulent effusion and required surgery, and a young pregnant woman underwent dialysis to manage acute kidney failure – all were Indigenous [97].
Factors contributing to high levels of APSGN in northern Australia
Humid climatic conditions in tropical northern Australia, the ubiquity of scabies and skin sores, and the relatively poor social and economic circumstances of many Indigenous people as identified elsewhere in this review, all contribute to the high rates of APSGN [34][52][81][82] [35][79][100]. In communities with high levels of scabies and skin sores, new strains spread very quickly with transmission facilitated by overcrowded living conditions and infrastructure problems in many remote communities [78][82]. The ease with which new strains of GAS are transmitted in families and communities is reflected in the rapid spread of APSGN [34]. Limited access to medical care, poor personal hygiene and inadequate environmental sanitation are also major contributing factors to the spread of the disease [34][78][82].
Prevention and management of ASPGN
There is no vaccine for GAS and no simple treatment for APSGN. In the absence of interventions, new cases can continue for several months [34]. Attempts to control APSGN outbreaks (Box 6) in Indigenous communities have frequently involved trying to stop the transmission of the bacteria by mass administration of parenteral penicillin to all children with any sign of skin sores [34][72][92][96][99], and sometimes regardless of whether they present with skin sores [76][79]. The targeted treatment of children with skin sores and household contacts has shown to be an effective intervention with the need for fewer injections (the injection is painful and reactions may occur) and a less labour-intensive response (that is correspondingly less likely to compromise routine services or miss high-risk children) [79][99]. Past trials employing more sensitive outbreak case definitions (‘sub-clinical’ cases now referred to as ‘probable cases’ and ‘clinical cases’ referred to as ‘possible cases’) have also resulted in an improvement in the screening of high risk communities [79].
Box 6. Current guidelines for the control of APSGN
Following the recording of an outbreak of APSGN, communities are advised to follow the guidelines for its control provided by the NT Centre for Disease Control (CDC) [34]. Currently, the guidelines define a community outbreak as either:
- two unrelated cases, either probable or confirmed that meet the clinical definition of APSGN (without laboratory confirmation) with onset within a week or each other, or
- one confirmed case and two probable cases in one month of each other that are not epidemiologically linked as a family, household or close contact.
Guidelines include community education and screening of all children up to the age of 15 years for skin sores, scabies and oedema. Management of infection currently involves hospital admission for major complications, coupled with the use of antibiotics to inhibit spread of the disease. If oedema is present, then the child is strongly advised to be assessed for manifestations of APSGN [34][52].
A recent community screen in April 2011 reported that 57% of targeted children were screened [73]. The successful access to the community largely resulted from the initial groundwork undertaken by the local health service, which facilitated community engagement beforehand as well as the expertise of the local Aboriginal health workers in identifying children and families. The use of ‘high quality’ rewards (in the case of this screening it was a plastic Sticky Hand developed by the Environmental Health Program) to attract more children to screening and treatment was also noted as a successful strategy to assist with the engagement of children about to receive injections.
In summary, good primary health care has been recognised as vital in the recognition and referral of APSGN, as early recognition of outbreaks and timely treatment is crucial in preventing the spread of GAS in the community and possible irreversible loss of kidney function [34][74]. The coexistence of high rates of GAS infections, APSGN and ESKD in northern Australia has raised the question of whether childhood APSGN could be linked with chronic kidney disease in later life [53][75][85]. With the aetiological link between scabies, streptococcal skin infections and outbreaks of APSGN well recognised [34][52][101], interventions for sustainable long-term improvements in skin health and the integration of environmental health, housing and hygiene programs with such interventions, are an essential part of the prevention, management and treatment of APSGN outbreaks [75][79][101][102][103][104]. An improvement in education, sanitation, and access to treatment is also a crucial step toward decreasing the incidence of glomerular disease [9][35]. Secondary and tertiary prevention depends on improved surveillance, screening to identify cases, and penicillin prophylaxis to eradicate streptococcal carriage and stem the spread of epidemic disease [52][96]. Prevention at this level will not only reduce rates of APSGN but may also contribute to a reduction in the incidence of ESKD [51][52][88].
Disorders of the urinary tract
Disorders of the urinary tract (specifically disorders of the bladder, ureters and urethra) generally present as clinically mild cases, but they have the potential to cause considerable morbidity, and may, on occasion, lead to severe kidney disease, including ESKD [35][36]. The potentially serious consequences associated with urinary tract disorders, coupled with their prevalence (particularly among women), highlight the public health significance of these conditions. Urinary tract infections (UTIs) (Box 7) are generally bacterial in origin, but, fungi, parasites and adenoviruses may also cause infection [36].
Box 7. Urinary tract infections (UTIs)
Most UTIs occur in the lower urinary tract as cystitis (in the bladder) and urethritis (in the urethra) [36][103]. Pyelonephritis, an upper UTI, is very serious as it may damage the kidneys. The urinary tract is normally free of bacteria and other micro-organisms, but these organisms can get into the bladder by coming up the urethra from the outside; sometimes they can enter from the kidneys or bloodstream [103]. Once in the bladder, bacteria can multiply very rapidly in warm urine. A UTI may be present despite few physical symptoms, but lower UTIs are typically associated with frequent, painful urination, and tenderness in the lower pelvic area [36]. Upper UTIs are associated with a range of clinical features, most commonly fever, back or loin pain, and chills [103]. Single episodes of UTI rarely have serious consequences, but recurrent or persistent infections may cause kidney damage when coupled with conditions such as diabetes [35]. Lower UTIs are easily treated, generally by a single dose or short course of antibiotics [36][103]. Upper UTIs usually require hospitalisation and administration of intravenous antibiotics. Antibiotics may also be administered to prevent recurrent infection.
UTIs are one of the most common reasons for people visiting a doctor about an infection [36]. Females are much more likely than males to contract UTIs, and other risk factors include age and sexual activity [35][105]. In Australia, about one in three adult women and one in 20 men will have a UTI in their lifetime. Women tend to be more susceptible to UTIs for several reasons, including the length of their urethra; the urethra is much shorter in females than in males, making it easier for microorganisms to travel to the bladder. Other factors include changes in hormonal levels (women are more likely to get an infection during certain times in their menstrual cycle, particularly just before a period and are also more susceptible during pregnancy). In women, the tissues of the urethra and bladder become thinner and drier with age, as well as after menopause or a hysterectomy: this also increases susceptibility to UTI [36][103].
Extent of UTIs among Indigenous people
The health impact of diseases of the urinary tract upon Indigenous people has received very little attention in recent years, but information from the 1980s suggests that the pattern of UTI exhibited among Indigenous people tends to differ from that observed among non-Indigenous people [105]. Epidemiological and anecdotal evidence from the 1980s indicates that UTIs were particularly common among Indigenous people [105][106]. A hospital-based study in Darwin found that Indigenous men and women had higher rates overall of UTI than did their non-Indigenous counterparts, but age-specific rates were greater among non-Indigenous people after the age of 60 years [105]. With the exception of children in their first year of life, UTIs occurred far more frequently among Indigenous females, with consistently high rates of infection until around 60 years of age. Indigenous males, on the other hand, were at greatest risk during infancy. Unfortunately, easily treated UTIs often remained undetected, particularly in Indigenous children, which increased the individual’s risk of developing more serious kidney disease [106]. Inadequate living conditions and poor environmental standards are thought to contribute to the high levels of UTI observed in some Indigenous communities [35][106].
Other data reveal that Indigenous women are more than twice as likely as non-Indigenous women to have a UTI during pregnancy [107][108]. A retrospective audit of medical records from rural and remote primary health care clinics in the NT of 268 pregnant women who gave birth in 2002 or 2003 revealed that 75% had at least one abnormal urinalysis during pregnancy (620 episodes were recorded overall) [107]. This is consistent with findings from earlier research that found almost 30% of Indigenous women sampled had a UTI during pregnancy, and 11% had an infection at the time of delivery [109]. Evidence from these studies suggests that screening, treatment and follow-up of UTIs among Indigenous people was often inadequate [106][107][108][109]. The occurrence of pyelonephritis (acute UTI that has reached the pelvis of the kidney) in women who had non-treated asymptomatic bacteriuria during pregnancy highlights the importance of screening, particularly for women living in rural and remote Indigenous communities.
Urolithiasis (kidney stones)
Urolithiasis refers to the formation of one or more pebble-like masses (commonly referred to as calculi or stones) in the kidney or urinary tract [31][32]. It is not a common public health problem within affluent populations in developed countries, but its prevalence and unusual presentation among some Indigenous children justifies its coverage here [37].
Extent of urolithiasis among Indigenous people
Very little attention has been directed at urolithiasis among Indigenous people since the 1990s, but the presence of a unique pattern among Indigenous children has been confirmed recently [37]. The findings of this study of Indigenous children presenting to the Darwin Hospital were very similar to those reported in the 1980s and 1990s [110][111][112][113][114][115][116].
The Darwin study found that Indigenous children living in remote areas of tropical and desert Australia have an unusual pattern of kidney stones [37]. Stones form early in life: the median age of presentation was 24 months with some stones recognised as early as 8 months of age. This pattern contrasts with that reported among non-Indigenous people and other populations of the developed world where the incidence is much higher among adults than children [37][110][115].
The recent research [37] is consistent with the reports from the 1980s and 1990s: Indigenous children with urolithiasis tend to come from desert regions of Australia, are more likely to be male, and are frequently less than 3 years of age [110][111][112][113][115]. Urolithiasis is rare among Indigenous children living in urban areas [37]. Children with stones commonly present with, or have a history of, failure to thrive, UTI, and/or recurrent infectious disease (particularly diarrhoea) [37][110][111][112][113][115]. The stones found in Indigenous children are rarely associated with anatomical or metabolic disorders and are commonly located in the upper urinary tract [110][111][113][115]. They are composed primarily of uric acid, and oxalate [110][111][112][113], similar to the ‘endemic’ stones typically found in paediatric populations from developing regions of the world where the disorder is prevalent [110].
The formation of calculi in non-Indigenous Australian children is uncommon [37][110][112]. When urolithiasis does occur in non-Indigenous children, it presents at a later age (usually mid to late childhood) and is generally attributable to a malformation of the urinary tract or a metabolic disorder [110][112]. A comprehensive review of patient records collected between 1972 and 1986 from the major paediatric referral hospital in WA reported that the number of Indigenous children presenting with urolithiasis was more than double that of non-Indigenous children [110]. A review of patients with urinary tract calculi admitted to the Urology Unit of the Adelaide Children’s Hospital between 1978 and 1987 estimated that 0.34% of Indigenous children under 10 years of age (based on 1981 census figures) suffered from the disease [112].
Factors contributing to urolithiasis among Indigenous people
Acidic stones are produced when there is a loss of alkaline bowel content (as a result of diarrhoea for example) [37]. The high rates of urolithiasis observed among some Indigenous children have been attributed to dietary factors, dehydration, endemic diarrhoea, recurrent infectious disease [37][110][111][112][113][114][115], hot, dry environmental conditions [110][111] and poor water quality [114]. These risk factors are intimately related to the familiar socioeconomic risk factors that underlie the high burden of disease suffered by Indigenous people generally.
Management and treatment of urolithiasis
Some stones appear to resolve spontaneously, but others might require surgical removal to avoid the risk of kidney damage [37]. The specific causes of kidney stones should be treated appropriately, but general treatment includes increased fluid intake (i.e. drinking water which is especially important for children during the weaning period and in for those warm climates), limited daily salt intake, moderate animal protein intake and medical treatment with alkali and thiazides (including sodium bicarbonate or potassium citrate [35][37]. Attention must be directed to the long-term public health implications and the need for preventive measures [112]. Housing, water and waste disposal systems are inadequate in many Indigenous communities and increase the risk of urolithiasis. Improvements in environmental conditions, specifically the provision of adequate drinking water and the eradication of poor living conditions, are therefore essential to reduce the incidence of kidney stones [37][114].
Management and treatment of CKD and ESKD
Kidney disease is expensive to treat and has a marked impact on the quality of life of those who suffer from the disease and of those who care for them [8][27]. Medical intervention is necessary to prevent deaths among individuals with CKD and ESKD. The current treatment option for ESKD involves KRT including dialysis (either haemodialysis (HD) or peritoneal dialysis (PD) (Box 8) and transplantation [9][29]. The aim of treatment for CKD (prior to reaching ESKD) is to delay progression of the disease. If CKD progresses to ESKD, KRT becomes necessary with the aim of providing ongoing quality and quantity of life rather than focusing the prevention of further deterioration of kidney function [9]. KRT cannot cure failing kidneys, but dialysis can enable survival. A further aim of treatment is to prevent added complications, such as cardiovascular issues [28][29]. Therefore, management of CKD also involves lifestyle modifications including: cessation of smoking; maintaining BMI below or equal to 25 kg/m2; engagement in regular physical activity; reducing alcohol and drug consumption; and maintaining a low salt diet [29].
Box 8. Dialysis: haemodialysis (HD) and peritoneal dialysis (PD)
Regular dialysis mechanically filters the blood to help maintain the functions normally performed by the kidneys [7][9]. There are two possible dialysis treatment options; haemodialysis (HD) and peritoneal dialysis (PD) [3][7][117]. Both treatment options aim to replace part of the (damaged) kidney’s function and both can be conducted from home and by the patient themselves [7][117].
Haemodialysis (HD)
In HD, blood is diverted from the body to a dialysis machine where it is filtered before being returned to the body [7][9]. As HD requires a costly machine to assist in the filtration of fluids outside the body [55], it is conducted at various locations, including hospital or dialysis units (located in tertiary hospitals), satellite centres (facilities linked with a tertiary centre renal unit) or managed carefully in the home [7][118]. The limited availability of such services outside of population centres, means that many Indigenous people are often forced to stay away from their land and families for long periods of time [55][118][119][120] and can mean that they die away from their people.
Peritoneal dialysis (PD)
In PD, the dialysis solution, containing a type of sugar that draws waste products and extra fluid out of the blood, is pumped into the abdomen and the blood filtered through the peritoneal membrane [9][55]. After a few hours the used solution containing waste and extra fluid is drained out of the body and replaced with fresh solution. The exchange takes about 30-45 minutes and can be undergone manually but requires dialysis exchanges 4-5 times daily; known as continuous ambulatory peritoneal dialysis (CAPD), or through the use of a machine which controls the timing of fluid exchanges while the patient sleeps; known as automated peritoneal dialysis (APD) [7][55][118]. Most people use PD at home, given its portability and convenience [7][118]. Both forms of treatment offer an alternative for people with CKD living in remote areas as it allows more independence and greater opportunity to return home more quickly [3][121].
Extent of dialysis among Indigenous people
A total of 187 Indigenous people commenced dialysis in Australia during 2009, a decrease from 249 in 2008 and 237 in 2007 [4]. In 2009, 1,174 prevalent dialysis patients in Australia were Indigenous; the level for Indigenous people was nearly five times that of their non-Indigenous counterparts (2,220 compared with 473 per 1,000,000 population) (Derived from [4]).
Based on information about hospitalisation, ‘care involving dialysis’ was most common for Indigenous people living in very remote areas (154 hospital separations per 1,000 population) [68]. Similarly, hospital separations relating to renal failure by socio-economic quintiles4 revealed that the ‘most disadvantaged’ quintile (representing the areas containing the 20% of the population with the least advantage/most disadvantage) had the highest separation rates dialysis. The residence in very remote areas of many Indigenous people requiring dialysis has important implications for how appropriate services are delivered.
Issues associated with relocation to undertake dialysis
When dialysis facilities are not available near to where the Indigenous patient lives, they are forced to move to regional centres or major cities to undertake dialysis. The lack of treatment available in remote areas and the limited availability of transplant facilities create geographical barriers to treatment with 78% of patients in remote areas having to relocate, compared with 39% of those who live in rural areas and 15% of urban Indigenous ESKD patients [6]. In recent years, research has revealed the significant biological, psychological, social and economic consequences to the forced relocation of Indigenous patients [122][123].
A study of Indigenous renal patients moving to Alice Springs from remote central Australian communities reported the socio-cultural alienation as extreme, debilitating and ultimately life threatening [124]. Recent research in WA reported similar findings for relocated Indigenous renal patients in that state [123]. The loneliness resulting from removal from family and land, including a sense of loss and disempowerment were considered worse than the illness (CKD) itself. Added to the stress of not being able to attend cultural events and obligations, the geographical isolation associated with treatment options often discourages Indigenous people from seeking treatment [6][123]. The impact of such issues is further highlighted by data reporting social causes as the second most important cause of death of Aboriginal and Torres Strait Islander people on dialysis (24%), after cardiac events (37%) [4].
In a bid to address poor treatment outcomes associated with relocation, efforts have been made to deliver self-care PD and HD dialysis services close to, or in, the home [125]. This means that Indigenous people remain in their own communities, with their own family and social supports, and the costs of re-housing and supporting relocated dialysis patients are avoided. However, challenges facing home HD in remote communities include being able to retain suitable dialysis partners/carers due to their requirements for attendance at cultural duties, suitable housing, changing social circumstances and communication problems (such as language barriers) [118][126]. In Queensland, three health zones have developed Renal Service Plans in recognition of Indigenous people as a priority population and in acknowledgement that many rural/remote areas of the state do not have ready access to hospital renal units or satellite units [127]. These plans include specific strategies to address kidney disease including health promotion, early detection, clinic management, kidney replacement services, workforce management and information support actions all specifically tailored to the needs of Indigenous people. Examples include the opening of the Mt Isa satellite service and the multi-user, self-care dialysis strategy established in the Toowoomba Renal Service. Satellite dialysis facilities are becoming more common in remote areas, but, in view of the low population density in many areas, access to treatment for people with ESKD is still an issue that requires dramatic changes in living circumstances [69][128]. Given ESKD relocation and treatment issues, it is considered good practice to allocate patients to the closest treatment facilities and to develop socio-culturally sensitive services for the specific needs of Indigenous patients.
In the search for alternative dialysis treatments, research has examined the use of Automated Wearable Artificial Kidneys (AWAK) [129]. Potential advantages proposed for this type of device include steady-state metabolic and fluid control, continuous regeneration of spent dialysate and the round-the-clock functionality of the device (mimicking the natural kidney). Such outcomes would allow patients to stay at home for their treatment and minimise interruption to their everyday activities. Despite AWAK’s potential to affect the wellbeing of CKD sufferers, especially Indigenous people in rural and remote areas, this type of treatment is still in a speculative stage of development.
Extent of HD among Indigenous people
Conducted in urban or regional clinics and hospitals, HD is the most common form of dialysis treatment for Indigenous people with ESKD [4][27][55][118][120]. In 2009, HD accounted for 81% of the new cases of Indigenous people commencing dialysis [4][40] and for the period 2007-08, hospitalisation data showed that Indigenous people were undergoing HD at 12.1 times the rate of non-Indigenous people5 [68]. In the same year, one in three hospital procedures for Indigenous peoples were for HD with recorded hospital separations higher in more remote areas.
A recent study comparing clinical outcomes and mortality rates of Indigenous people of Kimberley origin receiving HD treatment with other subsets of Indigenous HD patients and non-Indigenous Australians, revealed for the first time, similar mortality rates [130]. As a response to the new model of care for Kimberley patients undergoing HD in 2002, the study investigated outcomes for patients of Kimberley origin between January 2003 and December 2007, including patients receiving any form of KRT in any Australian location for that time period. An overall average of 70% of patients of Kimberley origin received HD treatment mainly provided by the Kimberley Satellite Dialysis Centre (KSDC), operated by an Aboriginal Community Controlled Health Service (ACCHS). The treatment was delivered at KSDC, at home in communities across the Kimberley, and at Derby Aboriginal Health Service. The study found that Indigenous people from the remote Kimberly region received HD treatment as good as elsewhere in the country. The authors felt that the excellent adherence of patients to care could be attributed to the delivery of quality services in culturally appropriate settings.
Extent of PD among Indigenous people
In 2009, the number of Indigenous people commencing peritoneal dialysis (PD) (35 patients) in Australia was less than in the previous two years [4]. By reducing the risks associated with relocation for HD, there are potential advantages of PD. However, as PD requires the patient to replace the dialysate bag several times a day with failure to change the bag resulting in life- threatening conditions, this places more responsibility on the patient to ensure correct management of the treatment [55]. Research has shown the correct management of PD treatment is impeded by various factors, such as infections and/or problems with the peritoneal dialysis catheter, home environment and child responsibility, constipation and treatment simply not suiting the individual [55]. Some anecdotal evidence also indicates that issues associated with changing the dialysate bag at home, such as facility issues with power and water, often leads to Indigenous people making the choice to undertake HD away from home. As previously discussed, this can create stress associated with having to relocate [123].
General issues associated with HD and PD
The multifaceted negative implications of HD and PD for Indigenous patients also reflect the differences between medical culture and that of Indigenous communities. Miscommunication between Indigenous patients and health care workers can occur because of differences in cultural and social beliefs on health, and in the understanding of the patient’s situation and ongoing treatment [43][131][132]. Such miscommunication is exacerbated for those Indigenous patients for whom English is a second language. Appropriate information and the associated understanding of the patient and their family are key for the patient – and their family and community – to make the important decisions about treatment choice [124][131][133].
Transplantation
Transplantation involves the implantation into a patient with ESKD of a kidney from either a living or recently deceased donor [7][9]. A recent systematic review of 110 international studies including a total of 1,961,904 participants with kidney failure revealed that transplantation was associated with substantial reductions in the risk of mortality and cardiovascular events [134]. It was also associated with clinically relevant improvements in quality of life, which improved further over time compared with treatment with different dialysis modalities. These findings confirm that kidney transplantation, when feasible, is the preferred therapeutic option for the management of CKD for patients and by health-care professionals [134][135]. Earlier studies revealed that the annual death rate of an age-matched population maintained by transplantation was reduced by about 80% beyond the first year compared with those remaining on dialysis [135][136]. The use of kidneys with no human leucocytes antigen (HLA) mismatches was associated with superior outcomes, but, unfortunately, completely matched kidneys account for a very small proportion of organs transplanted [137].
Extent of transplantation among Indigenous people
Transplantation is the optimal form of treatment for most ESKD patients, but there is a pronounced disparity between Indigenous people and non-Indigenous Australians for this treatment [117]. At the end of 2008, a far greater proportion of Indigenous people than non-Indigenous Australians were reliant on dialysis for KRT: only 12% of Indigenous people were living with a functioning transplant compared with 45% of other Australians6 [5]. Indigenous patients are both less likely than other Australians to receive a transplant and less likely to be wait-listed for a transplant [4][117][137]. Indigenous people receive transplants at approximately one-third the rate of other patients and those who do receive transplants wait longer for them [4].
In the four-year period 2006-2009, 2.1% of Indigenous people needing KRT received a transplant, compared with almost 11% of non-Indigenous people (Derived from [4]). For the same period, only 4% of all people on the waiting list were identified as Indigenous. Only 100 new transplant operations involved Indigenous recipients compared with 1,741 for non-Indigenous recipients. In most Australian states, the average wait for a kidney transplant from a deceased donor is about 4 years, and for some patients the wait is much longer [135]. Those Indigenous people who undergo transplantation usually need to travel large distances to reach medical centres [6][138].
Factors associated with access to kidney transplantation
Research investigating the reasons for the disparity between Indigenous ESKD patients and non-Indigenous patients receiving transplants (Box 9) reveals some key barriers to the accessibility of treatment [117]. System factors included: reduced likelihood of referral for transplant evaluation; failure to complete the comprehensive set of investigations required; and communication and education limitations. Individual factors included: greater likelihood of human leucocyte antigen (HLA) incompatibility; fear of their health status; refusal to leave land and community; higher rates of co-morbidities affecting acceptability for transplantation; and lower compliance to medical treatment as a result of communication problems and misunderstandings.
Box 9. Organ allocation in Australia
The allocation of organs, including kidneys, is a complex process that depends on a range of factors including medical need, urgency and the capacity of the recipient to benefit from the transplantation [139]. In Australia, allocation systems are underpinned by the principles of utility, equity and fairness. Kidneys matched by blood group and tissue compatibility are offered to the best suited recipient, regardless of where they live. There are only a few kidneys available for transplantation, so only patients deemed medically or otherwise appropriate are able to receive transplants. Patients are educated about the process to ensure informed decision making before being placed on the waiting list. When a donor organ becomes available or is identified, transplantation can take place [117].
A palliative care approach to the management and treatment of CKD and ESKD
Practices are being developed throughout Australia that contribute to the improvement of care, prevention and culturally appropriate management of CKD and ESKD [119][125][140][141][142][143][144][145][146][147]. Without the option of transplantation, dialysis is the only final stage treatment available for ESKD and, as such, it has the potential to be systematically coupled with palliative care units. For example; the Improving care for people with advanced chronic kidney disease: I’m OK project allocates Indigenous patients to a care coordinator who liaises with the different health professionals that the person sees [119]. The goal of the project is to reduce communication problems and enable close-to-home access to specialist health care in regional Aboriginal community controlled health services in the Northern Territory. The initiative offers a coordinated approach and contributes to the creation of strong links between the many different health staff who provide care to people with advanced CKD. The four-year long Palliative care for renal clients living in a remote setting project focuses on raising awareness of the palliative approach of the kidney health workforce and examines the palliative needs of clients with CKD [143]. The project aims to produce health resources and guidelines to accompany Indigenous renal patients through the end of their lives by providing support to make end-of-life decisions while also providing opportunity for health worker staff to embrace the palliative approach to care.
Prevention of ESKD
Kidney disease clearly has major medical and social implications for Indigenous people. The negative social consequences that accompany treatment and the high cost of tertiary level medical care illustrates the need for a comprehensive approach that addresses both the medical and socioeconomic dimensions of this growing problem [55][128].
Primary prevention of the social and economic conditions that underlie much of the Indigenous kidney health burden is a fundamental priority [53][54]. Secondary and tertiary measures, such as screening and pharmacological interventions, also promise to reduce the risk of serious kidney disease [148]. Screening for kidney disease has for some time been accomplished with simple, cheap and reliable techniques [61][149]. Such screening instruments may be readily incorporated within existing chronic disease screening protocols and coupled with interventions to modify the disease process [148].
An early example of a systematic treatment program implemented in the Tiwi Islands (off the coast of the NT) offering primary, secondary and tertiary prevention measures was designed to intervene in the disease process before individuals progress to ESKD. The program focused on vigorous blood pressure control and better metabolic management for people with diabetes and kidney disease, or with diabetes and high blood pressure and centred on the use of the long-acting angiotensin converting enzyme inhibitor, (ACEi) perindopril, an anti-hypertensive with cardiovascular and renal protective effects [150]. An early evaluation of the health outcomes of the program indicated that it had: slowed the progression of kidney disease; postponed kidney failure; reduced premature death; averted much cardiovascular morbidity; and diminished associated health care costs [21][151]. Progression to end-stage kidney failure had been reduced by one-half and there had also been a reduction in deaths from natural causes. The pattern of ESKD in the community had been reversed, with previously increasing rates of ESKD and natural death reduced [151].
The success of the program was attributed to a strong sense of community involvement and a partnership approach between the health workforce delivering the program and the community [152]. Specific facets of the program thought to have contributed to its success include:
- maximum involvement of local workers
- a community-based rather than clinic-based focus
- a collaborative, non-authoritarian approach
- involving participants in their own testing
- personalising health goals
- providing information not directives.
The program demonstrated that kidney disease can be easily diagnosed and its progression dramatically altered by available interventions [153]. It also showed that Indigenous people are interested in health issues and receptive to health messages; their willingness to participate enthusiastically and effectively in the long-term management of chronic disease led to demonstrable improvements in their kidney health [151]. Finally, the Tiwi model has shown that huge savings, in terms of both premature death and cost, are achievable if investments are made in community-based strategies for kidney disease [150]. It is estimated that the Tiwi program saved between $700,000 and $3.1 million in dialysis costs alone in its first three years of operation [153].
The need for comprehensive health care
In recent years, there has been significant research into the causes, consequences and management of ESKD in the Indigenous population [6][21][54][55][59][60][131][151][154][155][156][157]. A growing body of evidence demonstrates that some progress has been made [57], but it highlights, too, remaining gaps in the current health care approach. Consideration of the evidence as a whole provides guidance for the way forward, primarily indicating that Indigenous renal patients require, and deserve, effective, acceptable treatment. The current emphasis on complex, expensive, often problematic, hospital-based treatment should be balanced with comprehensive, community-based, preventive action to minimise the underlying causes of the problem [21][55][59][60][148].
The responsibility for reducing disparities in treatment rests primarily with the health care system and its providers [30][131][158][159]. This will require system-level changes, such as adequate funding for primary care, an adequate Indigenous health workforce, development of shared understandings about CKD, the use of high-quality and culturally appropriate educational resources, and improvements in the interface between primary care and specialist services. Prevention of the socioeconomic antecedents of kidney disease is inherent in such an approach, which calls for:
- sustained improvements in living and environmental conditions, education, infrastructure and health service [50][59][61]
- implementation of integrated, effective and well-resourced primary health care programs [21][59][69]
- primary health care measures to improve diet, control blood pressure and infections, maintain healthy adult weight, and to increase birth weight [30][50][59][61]
- systematic screening for early and established kidney disease [30][50][60][61][160][161]
- pharmacological intervention programs to slow disease development [35][60].
Evaluation is also a necessary component of any health program, and local ownership and management of health strategies for the Indigenous population is vitally important [55][148][162]. The added advantage of comprehensive action is that it will reduce not just kidney disease, but also other chronic and communicable conditions that underlie the much higher levels of mortality experienced by the Indigenous population [30][161][162]. It is within the capabilities of integrated socioeconomic and public health initiatives to quickly reduce kidney disease risk, and modify the existing disease profile, with potential savings in morbidity, mortality and health care costs [57][162].
Policies and strategies to improve kidney health
In Australia, the prevention and management of CKD and related kidney health issues is addressed more broadly under the banner of chronic diseases [163] and by way of strategies identifying areas for improvement in all aspects of CKD [69].
National strategies addressing CKD
The National chronic disease strategy (NCDS), released in 2006, includes information on CKD under the umbrella of chronic diseases [163]. The NCDS takes a holistic approach towards chronic diseases as these conditions often occur at the same time and their risk factors are similar. The strategy aims to better coordinate and manage chronic illnesses from prevention to palliation [163][164]. Further to this, the National chronic kidney disease strategy (NCKDS), proposed in 2006 by Kidney Health Australia, was released in response to the increasing burden of CKD in the Australian community [69]. Given the commonalities that exist between CKD and other conditions in the chronic non-communicable disease category (e.g. diabetes), the NCDS complements the NCKDS with synergies between the initiatives recognised within its framework and recommendations. Action areas across the strategies include: risk reduction, health promotion and illness prevention; early detection and early treatment and management; management of advanced CKD and self-management; and, more specific to CKD, priorities for dialysis, organ donation and transplantation [69][163].
With most of the underlying risk factors of CKD being primarily preventable, initiatives for risk reduction outlined by the NCDS and NCKDS include addressing the broad social determinants such as improvement of housing, education and employment opportunities, and the improvement, especially in remote areas, of affordable and healthy food choices and lifestyles [69][163]. Prevention and risk reduction approaches are applicable across the continuum of chronic disease care, aiming to prevent the diseases themselves, or their progression and associated complications and co-morbidities [163]. Early diagnosis offers the potential for both disease specific and non-specific interventions to slow disease progression. Even if progression cannot be slowed, patients who have been diagnosed early have better survival rates when commencing KRT [165]. Risk reduction seeks to prioritise the quality of life of the patient as well as the ability for self-management. Early treatment of CKD complications can also significantly improve the long-term patient outcomes [164]. In line with the current national strategy documents, self-management stems from the understanding that capacity building and training would enhance the ability of people to take more care for themselves (diminishing the impact of risk factors) and to control the health services in their communities (community-controlled services and increase of Aboriginal health workers) [163].
Recommendations and lessons learned in the delivery of services to improve kidney health
Addressing the needs of populations who are considered disadvantaged, including Aboriginal and Torres Strait Islander people, is of particular importance to both the NCDS and NCKDS [69][163]. Specifically, a key recommendation is the establishment of a national Aboriginal and Torres Strait Islander people chronic disease taskforce that transcends jurisdictional and community boundaries, as well as ensuring that all states/territories work towards providing equitable access to all forms of KRT for Indigenous peoples. In addition, the NCDS highlighted the importance of monitoring and improving data quality as part of the strategy towards reducing the burden of chronic disease [163]. In response to these needs, the National Centre for Monitoring Chronic Kidney Disease was established in 2007 at the Australian Institute of Health and Welfare (AIHW) [166]. In 2009, the Centre released its first publication An overview of chronic kidney disease in Australia 2009, which includes up-to-date national data on CKD [3]. Despite these developments, the various recommendations put forward by these initiatives have yet to be endorsed in the way of formal policies. Consequently, there is currently no comprehensive CKD strategy in Australia.
In 2010, the National Consumer Council of Kidney Health Australia7 released a position statement calling for the Australian Government to take action on the shortfall of services for CKD [128]. Similar in aims to the NCKDS [69], the position statement outlines changes to achieve optimal kidney health services and care in Australia. The main components of the position statement include: recognition of the extent of CKD as a major chronic condition; development of a national program to increase awareness and early detection of CKD; the funding and resourcing of the development and delivery of high quality information and education services in CKD; patient support programs and the training and promotion of these services in the community [128].
Recent work conducted by the Renal Division at the George Institute has involved several studies aimed at improving kidney health. One project – The central Australia renal study – commissioned by the Office for Aboriginal and Torres Strait Islander (OATSIH) within the Department of Health and Ageing (DoHA) – aimed to provide data and recommendations that would inform governments in the cross-jurisdictional region to make evidence based policy decisions that better meet the health and service needs of Aboriginal dialysis patients in the region [167]. Another project, commissioned by Kidney Health Australia, looking at the economic impact of the burden of CKD in Australia, aimed to establish the human and financial burden of CKD and to explore the cost-effectiveness of screening and intervention to prevent progression of disease [168]. Ongoing projects in the Division include the Improving access to kidney transplants (IMPAKT) project aimed at identifying Indigenous Australians’ barriers to accessing kidney transplantation, and proposing strategies to reduce disparities in Indigenous Australians’ access to transplantation [117][169]. In addition, findings from the Sharing the true stories (STTS), a longitudinal participatory action research (PAR) project (2001-05), provide support for solidifying policy in the kidney health arena [170]. Findings indicate that miscommunications and lack of shared understanding between Aboriginal client groups and health staff in renal and hospital services in the NT of Australia often limited patients’ opportunities and capacities to make informed choices about their health care. This suggests that, for there to be improvements in communication and health outcomes, fundamental change in the delivery of healthcare is required.
Other policies and one-off funding also continue to impact on the state of kidney health and related services in Australia. Under the umbrella of the Closing the gap strategy, which aims to reduce Indigenous disadvantages [171], the Council of Australian Governments (COAG) has shown commitment to reducing the factors that contribute to chronic disease [172][173]. Similar to the NCDS, COAG focuses on a preventive health approach through the strengthening of primary health care as well as focussing on sustainability and cultural appropriateness [174]. In addition, the Australian Government has funded specific initiatives to reduce the incidence and spread of kidney diseases including: grants into kidney disease research via the National Health and Medical Research Council (NHMRC); the construction of a renal dialysis unit at the North Lakes Health Precinct in Queensland; funding towards improving the access of dialysis services in the Kimberley region of WA and for remote communities in the Northern Territory; and development of the Chronic Kidney Disease Monitoring Centre [175].
Concluding comments
Disorders of the kidneys and urologic system pose a significant, and frequently serious, public health threat for many Indigenous people [6][7][29]. ESKD, which underlies much of the renal morbidity and mortality seen in Indigenous communities, is the clear priority for the health system, but recurring epidemics of APSGN in northern Australia, the suspected high levels of UTIs, and the unusual epidemiology of urolithiasis also require appropriate attention. Continuing high rates of ESKD, the negative social consequences that accompany treatment, and the high cost of tertiary level medical care all illustrate the immediate need for a comprehensive health care approach that addresses both the medical and socioeconomic dimensions of this major problem [30][131][159].
A range of biopsychosocial issues underlie the generally poor health status of many Indigenous people. Poverty, poor living conditions, limited access to medical care, and inadequate environmental sanitation contribute to high rates of kidney health disorders in many Indigenous communities [7][49][50]. The prevention, management and control of renal-urologic disorders will depend not only on effective, acceptable medical and surgical treatment, but, importantly, on preventive action to address the poor socioeconomic circumstances that underlie these conditions. Without adequate forward planning that considers service needs, service availability, and workforce projections, however, there will not be adequate resources to provide minimum standards of care for the growing number of Indigenous people dependant on dialysis [4][153]. A comprehensive approach that addresses both the medical and socioeconomic dimensions of these health conditions is an immediate priority.
References
- Australian Bureau of Statistics (2006) National Aboriginal and Torres Strait Islander Health Survey: Australia, 2004-05. Canberra: Australian Bureau of Statistics
- Australian Bureau of Statistics, Australian Institute of Health and Welfare (2008) The health and welfare of Australia’s Aboriginal and Torres Strait Islander Peoples 2008. Canberra: Australian Bureau of Statistics and Australian Institute of Health and Welfare
- Australian Institute of Health and Welfare (2009) An overview of chronic kidney disease in Australia, 2009. Canberra: Australian Institute of Health and Welfare
- Australia and New Zealand Dialysis and Transplant Registry (2010) The thirty third report: Australia and New Zealand Dialysis and Transplant Registry: 2010. Adelaide: Australia and New Zealand Dialysis and Transplant Registry
- Australian Institute of Health and Welfare (2011) Aboriginal and Torres Strait Islander health performance framework 2010: detailed analyses. Canberra: Australian Institute of Health and Welfare
- Preston-Thomas A, Cass A, O’Rourke P (2007) Trends in the incidence of treated end-stage kidney disease among Indigenous Australians and access to treatment. Australian and New Zealand Journal of Public Health; 31(5): 419-421
- Australian Institute of Health and Welfare (2011) Chronic kidney disease in Aboriginal and Torres Strait Islander people 2011. Canberra: Australian Institute of Health and Welfare
- Kidney Health Australia (2010) The economic impact of end-stage kidney disease in Australia – projections to 2020. Melbourne: Kidney Health Australia
- National Kidney and Urologic Diseases Information Clearinghouse (2009) The kidneys and how they work. Retrieved 2009 from http://kidney.niddk.nih.gov/kudiseases/pubs/pdf/yourkidneys.pdf
- McDonald S, Maguire GP, Hoy W (2003) Renal function and cardiovascular risk markers in a remote Australian Aboriginal community. Nephrology Dialysis Transplantation; 18(8): 1555-1561
- Hoy WE, Mathews JD, McCredie DA, Pugsley DJ, Hayhurst BG, Rees M, Kile E, Walker KA, Wang Z (1998) The multidimensional nature of renal disease: rates and associations of albuminuria in an Australian Aboriginal community. Kidney International; 54(4): 1296-1304
- Shephard MDS, Allen GG, Barratt LJ, Barbara JAJ, Paizis K, McLeod G, Brown M, Vanajek A (2003) Albuminuria in a remote South Australian Aboriginal community: results of a community-based screening program for renal disease. Rural and Remote Health; 3(1): 156 Retrieved from http://www.rrh.org.au/publishedarticles/article_print_156.pdf
- Spiers BF (2009) Antecedents of chronic kidney disease in Aboriginal offenders in New South Wales prisons: Dr Ross Ingram Memorial Essay Competition. Medical Journal of Australia; 190(10): 524-526
- Hoy WE, White AV, Dowling A, Sharma SK, Bloomfield H, Tipiloura BT, Swanson CE, Mathews JD, McCredie DA (2012) Post-streptococcal glomerulonephritis is a strong risk factor for chronic kidney disease in later life. Kidney International; 81(2): 1026-1032
- Haysom L, Williams R, Hodson E, Lopez-Vargas P, Roy LP, Lyle D, Craig JC (2009) Risk of CKD in Australian Indigenous and non-Indigenous children: a population-based cohort study. American Journal of Kidney Diseases; 53(2): 229-237
- Haysom L, Williams R, Hodson EM, Lopez-Vargas PA, Roy LP, Lyle DM, Craig JC (2009) Natural history of chronic kidney disease in Australian Indigenous and non-Indigenous children: a 4-year population-based follow-up study. Medical Journal of Australia; 190(6): 303-306
- Haysom L, Williams R, Hodson E, Lopez-Vargas P, Roy LP, Lyle D, Craig JC (2008) Diagnostic accuracy of urine dipsticks for detecting albuminuria in Indigenous and non-Indigenous children in a community setting. Pediatric Nephrology; 24(2): 323-331
- Haysom L, Williams R, Hodson E, Roy LP, Lyle D, Craig JC (2007) Early chronic kidney disease in Aboriginal and non-Aboriginal Australian children: remoteness, socioeconomic disadvantage or race?. Kidney International; 71(8): 787-794
- The SEARCH Investigators (2010) The study of environment on Aboriginal resilience and child health (SEARCH): study protocol. BMC Public Health; (10): 287 Retrieved from http://www.biomedcentral.com/content/pdf/1471-2458-10-287.pdf
- Maple-Brown LJ, Lawton PD, Hughes JT, Sharma SK, Jones GRD, Ellis AG, Hoy W, Cass A, MacIsaac RJ, Sinha AK, Thomas MAB, Piers LS, Ward LC, Drabsch K, Panagiotopoulos S, McDermott R, Warr K, Cherian S, Brown A, Jerums G, O’Dea K (2010) Study protocol – accurate assessment of kidney function in Indigenous Australians: aims and methods of the eGFR Study. BMC Public Health; (10): 80 Retrieved from http://www.biomedcentral.com/content/pdf/1471-2458-10-80.pdf
- Hoy W, Kelly A, Jacups S, McKendry K, Baker P, MacDonald S, Wang Z, Punguatji N, Kerinauia J, Tipiloura E, Tipiloura E, Harrison C (1999) Stemming the tide: reducing cardiovascular disease and renal failure in Australian Aborigines. Australian and New Zealand Journal of Medicine; 29(3): 480-483
- Hoy W (1996) Renal disease in Australian Aboriginals: community health programs are critical to containing the renal disease epidemic [editorial]. Medical Journal of Australia; 165: 126-127
- Australian Institute of Health and Welfare (2010) Chronic kidney disease hospitalisations in Australia 2000–01 to 2007–08. Canberra: Australian Institute of Health and Welfare
- Australian Institute of Health and Welfare (2011) Australian hospital statistics 2009-10. Canberra: Australian Institute of Health and Welfare
- Australian Bureau of Statistics (2011) Causes of death, Australia, 2009. Canberra: Australian Bureau of Statistics
- Australian Institute of Health and Welfare (2008) Aboriginal and Torres Strait Islander health performance framework, 2008 report: detailed analyses. Canberra: Australian Institute of Health and Welfare
- Australian Bureau of Statistics (2010) The health and welfare of Australia’s Aboriginal and Torres Strait Islander peoples, 2010. Canberra: Australian Bureau of Statistics
- Australian Institute of Health and Welfare (2008) Australia’s health 2008: the eleventh biennial health report of the Australian Institute of Health and Welfare. Canberra: Australian Institute of Health and Welfare
- Chronic kidney disease (CKD) management in general practice (2009) Kidney Health Australia
- Kidney Health Australia (2011) Two of a KinD (kidneys in diabetes) : the burden of diabetic kidney disease and the cost effectiveness of screening people with type 2 diabetes for chronic kidney disease. Melbourne: Kidney Health Australia
- Pearle MS, Calhoun E, Curhan GC (2007) Urolithiasis. In: Litwin MS, Saigal CS, eds. Urologic diseases in America. Washington, DC: National Institutes of Health: 281-319
- Kidney stones (2009) Kidney Health Australia
- Rodriguez-Iturbe B, Musser JM (2008) The current state of poststreptococcal glomerulonephritis. Journal of the American Society of Nephrology; 19: 1855-1864
- Centre for Disease Control (2010) Northern Territory guidelines for acute post-streptococcal glomerulonephritis. Casuarina: Department of Health and Community Services, Northern Territory Government
- Dirks J, Remuzzi G, Horton S, Schieppati A, Rizvi SAH (2006) Diseases of the kidney and the urinary system. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, eds. Disease control priorities in developing countries, 2nd edition. Washington (DC): World Bank: 695-706
- Urinary tract infections (2009) Kidney Health Australia
- Carson PJ, Brewster DR (2003) Unique pattern of urinary tract calculi in Australian Aboriginal children. Journal of Paediatrics and Child Health; 39(5): 325-328
- McDonald S, Chang S, Excell L, eds (2007) Australia and New Zealand Dialysis and Transplant Registry: the thirtieth report. Adelaide: Australia and New Zealand Dialysis and Transplant Registry
- McDonald S, Excell L, Livingston B (2008) Australia and New Zealand Dialysis and Transplant Registry: the thirty first report. Adelaide: Australia and New Zealand Dialysis and Transplant Registry
- McDonald S, Excell L, Livingston B (2009) The thirty second report – Australia and New Zealand Dialysis and Transplant Registry 2009. Adelaide: Australia and New Zealand Dialysis and Transplant Registry
- Saggers S, Gray D (1991) Aboriginal health and society: the traditional and contemporary Aboriginal struggle for better health. North Sydney: Allen and Unwin
- Carson B, Dunbar T, Chenhall RD, Bailie R, eds. (2007) Social determinants of Indigenous health. Crows Nest, NSW: Allen and Unwin
- Thomson N, ed. (2003) The health of Indigenous Australians. South Melbourne: Oxford University Press
- Kamien M (1980) The Aboriginal Australian experience. In: Stanley NF, Joske RA, eds. Changing disease patterns and human behaviour. London: Academic Press: 254-270
- Moodie PM (1981) Australian Aborigines. In: Trowell H C, Burkitt DP, eds. Western diseases: their emergence and prevention. London: Edward Arnold: 154-167
- Brown A, Walsh W, Lea T, Tonkin A (2005) What becomes of the broken hearted?: coronary heart disease as a paradigm of cardiovascular disease and poor health among health among Indigenous Australians [editorial]. Heart, Lung and Circulation; 14(3): 158-162
- Wilkinson R, Marmot M (2003) Social determinants of health: the solid facts. Denmark: World Health Organization
- Thomson N, Brooks J (2003) Cardiovascular disease. In: Thomson N, ed. The health of Indigenous Australians. South Melbourne: Oxford University Press: 186-206
- Australian Health Ministers’ Advisory Council (2011) Aboriginal and Torres Strait Islander health performance framework: 2010 report. Canberra: Office for Aboriginal and Torres Strait Islander Health, Department of Health and Ageing
- Cass A, Cunningham J, Snelling P, Wang Z, Hoy W (2004) Exploring the pathways leading from disadvantage to end-stage renal disease for Indigenous Australians. Social Science & Medicine; 58(4): 767-785
- Singh GR (2009) Glomerulonephritis and managing the risks of chronic renal disease. Pediatric Clinics of North America; 56(6): 1363-1382
- Centre for Disease Control (2010) Healthy Skin Program: guidelines for community control of scabies, skin sores and crusted scabies in the Northern Territory. Darwin: Northern Territory Department of Health and Families
- White A, Wong W, Sureshkumur P, Singh G (2010) The burden of kidney disease in Indigenous children of Australia and New Zealand, epidemiology, antecedent factors and progression to chronic kidney disease. Journal of Paediatrics and Child Health; 46(9): 504-509
- Cass A, Cunningham J, Hoy W (2002) The relationship between the incidence of end-stage renal disease and markers of socioeconomic disadvantage. New South Wales Public Health Bulletin; 13(7): 147-151
- Preece C (2010) Developing a model of care to improve the health and well-being for Indigenous people receiving renal dialysis treatment. Master of Applied Science (Research) thesis, Queensland University of Technology: Brisbane
- Steering Committee for the Review of Government Service Provision (2011) Report on government services 2011: Indigenous compendium. Canberra: Productivity Commission
- Hoy WE, Kincaid-Smith P, Hughson MD, Fogo A, Sinniah R, Dowling J, Samuel T, Mott SA, Douglas-Denton RN, Bertram JF (2010) CKD in Aboriginal Australians. American Journal of Kidney Diseases; 56(5): 983–993
- Li Z, McNally L, Hilder L, Sullivan EA (2011) Australia’s mothers and babies 2009. Canberra: Australian Institute of Health and Welfare
- Hoy W (1998) Renal disease as a metaphor: towards a more integrated view of Aboriginal health. Clinical and Experimental Pharmacology and Physiology; 25(12): 1038-1042
- Spencer JL, Silva DT, Snelling P, Hoy WE (1998) An epidemic of renal failure among Australian Aboriginals. Medical Journal of Australia; 168(11): 537-541
- Thomas MAB (1998) Kidney disease in Australian Aboriginals: time for decisive action [editorial]. Medical Journal of Australia; 168(11): 532-533
- Australian Bureau of Statistics (2006) National Health Survey: summary of results, Australia, 2004-05. Canberra: Australian Bureau of Statistics
- Australia and New Zealand Dialysis and Transplant Registry (2012) ANZDATA structure. Retrieved 2012 from http://www.anzdata.org.au/v1/structure.html
- End stage renal disease notifications, by Indigenous status, age, jurisdiction and year [1995 to 2009, unpublished] (2011) Australian and New Zealand Dialysis and Transplant Registry
- Australian Bureau of Statistics (2008) Australian Demographic Statistics: June quarter 2008. Canberra: Australian Bureau of Statistics
- Australian Bureau of Statistics (2001) Australian demographic statistics: June quarter 2001. Canberra: Australian Bureau of Statistics
- Australian Bureau of Statistics (2009) Experimental estimates and projections, Aboriginal and Torres Strait Islander Australians: Projected population, Aboriginal and Torres Strait Islander Australians, Australia, states and territories, 2006-2021 series [data cube]. Retrieved 8 September 2009 from http://www.abs.gov.au/ausstats/subscriber.nsf/log?openagent&32380ds002_2006.srd&3238.0&Data%20Cubes&E79A5308D989173FCA25762A001CE1C3&0&1991%20to%202021&08.09.2009&Latest
- Australian Institute of Health and Welfare (2009) Australian hospital statistics 2007-08. Canberra: Australian Institute of Health and Welfare
- Kidney Health Australia (2006) National chronic kidney disease strategy. Melbourne: Kidney Health Australia
- Andreasyan K, Hoy WE (2010) Recent patterns in chronic disease mortality in remote living Indigenous Australians. BMC Public Health; 10: 483 Retrieved 16 August 2010 from http://dx.doi.org/10.1186/1471-2458-10-483
- Geetha D (2010) Glomerulonephritis, Poststreptococcal: overview. Retrieved from http://emedicine.medscape.com/article/240337-overview
- Scrace M, Koko K (2006) An outbreak of acute post-streptococcal glomerulonephritis in remote Far North Queensland. Australian Journal of Rural Health; 14(4): 160-163
- Mansfield K, Gunn J, Wilson N, Scott L (2011) Acute post-streptococcal glomerulonephritis and opportunistic trachoma screening in an Indigenous community in the Northern Territory, 2011. Northern Territory Disease Control Bulletin; 18(4): 8-12
- Isbel NM (2005) Glomerulonephritis – management in general practice. Australian Family Physician; 34(11): 907-9, 911-3
- Marshall C, Taylor C (2008) Acute post-streptococcal glomerulonephritis in the Northern Territory 2008. Northern Territory Disease Control Bulletin; 15(3): 1-5
- Scott L (2004) Acute post-streptococcal glomerulonephritis in a remote community. Northern Territory Disease Control Bulletin; 11(3): 7-8
- Whitehead BD, Smith HV, Nourse C (2010) Invasive group A streptococcal disease in children in Queensland. Epidemiology and Infection; 139(4): 623-628
- Holt DC, McCarthy JS, Carapetis JR (2010) Parasitic diseases of remote Indigenous communities in Australia. International Journal for Parasitology; 40(10): 1119–1126
- Binns P, Markey P, Krause V (2005) An outbreak of acute post-streptococcal glomerulonephritis in a remote Aboriginal community in 2005. Northern Territory Communicable Diseases Bulletin; 12(2): 12-15
- Andrews RM, Kearns T, Connors C, Parker C, Carville K, Currie BJ, Carapetis JR (2009) A regional initiative to reduce skin infections amongst Aboriginal children living in remote communities of the Northern Territory, Australia. PLoS Neglected Tropical Diseases; 3(11): 1-9
- McMeniman E, Holden L, Kearns T, Clucas DB, Carapetis JR, Currie BJ, Connors C, Andrews RM (2011) Skin disease in the first two years of life in Aboriginal children in East Arnhem Land. Australasian Journal of Dermatology; 52(4): 270–273
- Hudson S (2011) One disease at a time: eradicating scabies in east Arnhem Land. Policy; 27(2): 23-25
- Marshall CS, Cheng AC, Markey PG, Towers RJ, Richardson LJ, Fagan PK, Scott L, Krause VL, Currie BJ (2011) Acute post-streptococcal glomerulonephritis in the Northern Territory of Australia: a review of 16 years data and comparison with the literature. American Society of Tropical Medicine and Hygiene; 85(4): 703-710
- Clucas DB, Carville KS, Connors C, Currie B, Carapetis J, Andrews R (2008) Disease burden and health-care clinic attendances for young children in remote Aboriginal communities of northern Australia. Bulletin of the World Health Organization; 86(4): 275-281
- Steer AC, Danchin MH, Carapetis JR (2007) Group A streptococcal infections in children. Journal of Paediatrics and Child Health; 43(4): 203-213
- Gogna NK, Nossar V, Walker AC (1983) Epidemic of acute poststreptococcal glomerulonephritis in Aboriginal communities. Medical Journal of Australia; 1: 64-66
- Devanesen D, Bernard E, Stokes ML, Daby J, Withnall K, Falls G, Peach H (1987) Lessons from an outbreak of glomerulonephritis in an Aboriginal community. In: Annual Report Menzies School of Health Research. : : 86-89
- White AV, Hoy WE, McCredie DA (2001) Childhood post-streptococcal glomerulonephritis as a risk factor for chronic renal disease in later life. Medical Journal of Australia; 174: 492-496
- Hoy W, Wang Z, Vanbuynder P, Baker P, McDonald SM, Mathews J (2001) The natural history of renal disease in Australian Aboriginies: part 2, Albuminuria predicts natural death and renal failure. Kidney International; 60: 249-256
- Singh GR, Hoy WE (2004) Kidney volume, blood pressure, and albuminuria: findings in an Australian Aboriginal community. American Journal of Kidney Diseases; 43(2): 254-259
- Maple-Brown LJ, Cunningham J, Hodge AM, Weeramanthri T, Dunbar T, Lawton PD, Zimmet PZ, Chadban SJ, Polkinghorne KR, Shaw JE, O’Dea K (2011) High rates of albuminuria but not of low eGFR in urban Indigenous Australians: the DRUID Study. BMC Public Health; 11: 346 Retrieved from http://dx.doi.org/10.1186/1471-2458-11-346
- Williams S, Markey P (2005) Acute post streptococcal glomerulonephritis in a remote community. Northern Territory Communicable Diseases Bulletin; 12(2): 16-18
- Streeton C (1995) A review of the epidemiology of acute poststreptococcal glomerulonephritis and acute rheumatic fever in far North Queensland. In: Streeton C, ed. Master of Applied Epidemiology bound volume. Canberra: National Centre for Epidemiology and Population Health, Australian National University: 211-218
- McDonald MI, Towers RJ, Andrews R, Benger N, Fagan P, Currie BJ, Carapetis JR (2008) The dynamic nature of group A streptococcal epidemiology in tropical communities with high rates of rheumatic heart disease. Epidemiology and Infection; 136(4): 529-539
- McDonald MI, Towers RJ, Fagan P, Carapetis JR, Currie BJ (2007) Molecular typing of Streptococcus pyogenes from remote Aboriginal communities where rheumatic fever is common and pyoderma is the predominant streptococcal infection. Epidemiology and Infection; 135(8): 1398–1405
- Streeton C, Hanna J, Messer R, Merianos A (1995) An epidemic of acute post-streptococcal glomerulonephritis among Aboriginal children. Journal of Paediatrics and Child Health; 31(3): 245-248
- Streeton C, Hanna J (1995) Acute poststreptococcal glomerulonephritis and acute rheumatic fever in far North Queensland, 1994. Communicable Diseases Intelligence; 19(6): 137-140
- Carapetis J (1995) An outbreak of acute poststreptococcal glomerulonephritis in an Aboriginal community. Communicable Diseases Intelligence; 19(6): 140-142
- Johnston F, Carapetis J, Patel MS, Wallace T, Spillane P (1999) Evaluating the use of penicillin to control outbreaks of acute poststreptococcal glomerulonephritis. Pediatric Infectious Disease Journal; 18(4): 327-332
- McDonald M, Towers R, Fagan P, McKinnon M, Benger N, Andrews R, Currie BJ, Carapetis J (2006) Recovering streptococci from the throat, a practical alternative to direct plating in remote tropical communities. Journal of Clinical Microbiology; 44(2): 547-552
- La Vincente S, Kearns T, Connors C, Cameron S, Carapetis J, Andrews R (2009) Community management of endemic scabies in remote Aboriginal communities of northern Australia: low treatment uptake and high ongoing acquisition. PLoS Neglected Tropical Diseases; 3(5): e444 Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2680947
- Gilmore SJ (2011) Control strategies for endemic childhood scabies. PLoS ONE; 6(1): e15990 Retrieved 25 January 2011 from http://www.plosone.org/article/fetchObjectAttachment.action;jsessionid=EC322D086620F361C24C7F8EA8A8C38E.ambra02?uri=info%3Adoi%2F10.1371%2Fjournal.pone.0015990&representation=PDF
- Brown D, Edwards H, eds. (2007) Medical-surgical nursing : assessment and management of clinical problems. Marrickville, N.S.W.: Elsevier Australia
- Atkins RC (2001) How bright is their future? Post-streptococcal glomerulonephritis in Indigenous communities in Australia [editorial]. Medical Journal of Australia; 174: 489-490
- Assumpcao C (1988) Some urinary tract disease in Australian Aboriginal inpatients in 1980. Australian and New Zealand Journal of Medicine; 18(1): 17-20
- Williams R, Allam B (1992) Aboriginal renal disease awareness. Aboriginal and Islander Health Worker Journal; 16(1): 4-5
- Bookallil M, Chalmers E, Andrew B (2005) Challenges in preventing pyelonephritis in pregnant women in Indigenous communities. Rural and Remote Health; 5(3): 395 Retrieved from http://www.rrh.org.au/publishedarticles/article_print_395.pdf
- de Costa C, Child A (1996) Pregnancy outcomes in urban Aboriginal women. Medical Journal of Australia; 164(9): 523-526
- Schultz R, Read AW, Straton AY, Stanley FJ, Morich P (1991) Genitourinary tract infections in pregnancy and low birth weight: case-control study in Australian Aboriginal women. British Medical Journal; 303: 1369-1373
- Jones TW, Henderson TR (1989) Urinary calculi in children in Western Australia: 1972-86. Australian Paediatric Journal; 25(2): 93-95
- Adsett DB (1982) Urinary calculi in children of the Kimberley region of Western Australia. Australian Paediatric Journal; 18(4): 283-285
- Cassey JG, Ahmed S (1989) Urinary tract calculi in Aboriginal children. Australian Paediatric Journal; 25(6): 363-365
- Thambi Dorai C, Dewan P, Boucaut H, Ehrlich J (1994) Urolithiasis in Australian Aboriginal children. Australian and New Zealand Journal of Surgery; 64(2): 99-101
- Williams W, Nicholas J, Nungurrayi P, Napurrula C (1996) Paediatric urolithiasis in a remote Australian Aboriginal community. Journal of Paediatrics and Child Health; 32(4): 344-346
- Wisniewski ZS, Brockis JG, Ryan GD (1981) Urinary bladder stones in Aboriginal children. Australian and New Zealand Journal of Surgery; 51(3): 292-295
- Farago C (1987) Overinvestigation of Aboriginal children with urinary tract infection? [letter]. Medical Journal of Australia; 147(10): 521
- Devitt J, Devitt J, Cass A, Cunningham J, Preece C, Anderson K, Snelling P (2008) Improving access to kidney transplants (IMPAKT): a detailed account of a qualitative study investigating barriers to transplant for Australian Indigenous people with end-stage kidney disease. BMC Health Services Research; 8: 31 Retrieved 4 February 2008 from http://www.biomedcentral.com/1472-6963/8/31
- Agar JWM, Hawley CM, George CRP, Mathew TH, McDonald SP, Kerr PG (2010) Home haemodialysis in Australia — is the wheel turning full circle?. Medical Journal of Australia; 192(7): 403-406
- Northern Territory Department of Health and Families (2010) I’m OK: improving care for people with advanced chronic kidney disease. The Chronicle; 16(1): 1-4
- Prout S, Yap M (2010) Indigenous temporary mobilities and service delivery in regional service centres: a West Kimberley case study. Canberra: Centre for Aboriginal Economic Policy Research
- Carruthers D, Warr K (2004) Supporting peritoneal dialysis in remote Australia. Nephrology; 9(Supplement 4): s129-s133
- Department of Health and Community Services (2005) Renal services strategy. Darwin: Department of Health and Community Services, Northern Territory Government
- Le B, Kickett M (2009) Dislocation and dialysis in Aboriginal patients with renal failure. Aboriginal and Islander Health Worker Journal; 33(4): 10-13
- Devitt J, McMasters A (1998) Living on medicine: a cultural study of end-stage renal disease among Aboriginal people. Alice Springs: IAD Press
- Barnes J, Marfan J, Brown D (2010) The self-care dialysis program. The Chronicle; 16(1): 10-11
- Villarra A, Warr K (2004) Home haemodialysis in remote Australia. Nephrology; 9(Supplement 4): s134-s137
- Standing Committee on Aboriginal and Torres Strait Islander Health, Statistical Information Management Committee (2006) National summary of the 2003 and 2004 jurisdictional reports against the Aboriginal and Torres Strait Islander health performance indicators. Canberra: Australian Institute of Health and Welfare
- National Consumer Council of Kidney Health Australia (2010) The impact of kidney disease and what government should do about it. Melbourne: Kidney Health Australia
- David BNL, Roberts M (2008) A peritoneal-based automated wearable artificial kidney. Clinical and Experimental Nephrology; 12(3): 171-180
- Marley JV, Dent HK, Wearne M, Fitzclarence C, Nelson C, Siu K, Warr K, Atkinson D (2010) Haemodialysis outcomes of Aboriginal and Torres Strait Islander patients of remote Kimberley region origin. Medical Journal of Australia; 193(9): 516-520
- Anderson K, Devitt J, Cunningham J, Preece C, Cass A (2008) “All they said was my kidneys were dead”: Indigenous Australian patients’ understanding of their chronic kidney disease. Medical Journal of Australia; 189(9): 499-503
- Lowe M, Kerridge IH, Mitchel KR (1995) ‘These sorts of people don’t do very well’: race and allocation of health care resources. Journal of Medical Ethics; 21(6): 356–360
- MacMahon J, Stewart S (2010) Your future: advanced care planning for renal clients. The Chronicle; 16(1): 17
- Tonelli M, Wiebe N, Knoll G, Bello A, Browne S, Jadhavb D, Klarenbacha S, Gillc J (2011) Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. American Journal of Transplantation; 11(10): 2093-2109
- Mathew T, Faull R, Snelling P (2005) The shortage of kidneys for transplantation in Australia: editorial. Medical Journal of Australia; 182(5): 204-205
- McDonald SP, Russ GR (2002) Survival of recipients of cadaveric kidney transplants compared with those receiving dialysis treatment in Australia and New Zealand, 1991–2001. Nephrology Dialysis Transplantation; 17(12): 2212-2219
- Cass A, Cunningham C, Snelling P, Wang Z, Hoy W (2003) Renal transplantation for Indigenous Australians: identifying the barriers to equitable access. Ethnicity & Health; 8(2): 111-119
- Cass A, Cunningham J, Wang Z, Hoy W (2001) Regional variation in the incidence of end-stage renal disease in Indigenous Australians. Medical Journal of Australia; 175: 24-27
- How the waiting lists operate: organ donation (2009) Australian Organ and Tissue Donation and Transplantation Authority
- Johnston R (2010) Chronic kidney disease: a coordinated approach. The Chronicle; 16(1): 5
- Amega B, Bowen E (2010) Together – no more them and us!. The Chronicle; 16(1): 6-7
- Race K, Nash F (2010) NT Australian Better Health Initiative (ABHI) project: primary care integration. The Chronicle; 16(1): 8-9
- Stewart S (2010) Palliative care for renal clients living in a remote setting. The Chronicle; 16(1): 12-13
- Program of Experience in the Palliative Approach learning guide for Aboriginal and Torres Strait Islander Health Workers (2011) Program of Experience in the Palliative Approach
- Stewart S (2010) “Palliative care for sick kidneys” education resource. The Chronicle; 16(1): 18
- Murphy S (2010) A journey home to country. The Chronicle; 16(1): 16
- Gorham G (2010) Renal Indigenous resources project. The Chronicle; 16(1): 19
- Gorham G (2003) Prevention and treatment options for renal disease in the Northern Territory (with particular reference to the Barkly region). Casuarina: Cooperative Research Centre for Aboriginal and Tropical Health
- Couzos S, Thomas M, Cass A (2008) Chronic kidney disease. In: Couzos S, Murray R, eds. Aboriginal primary health care: an evidence-based approach. 3rd ed. South Melbourne: Oxford University Press: 575-621
- Menzies School of Health Research (2000) Menzies School of Health Research 1999-2000 Annual Report. Darwin: Menzies School of Health Research
- Hoy WE, Baker PR, Kelly AM, Wang Z (2000) Reducing premature death and renal failure in Australian Aboriginals: a community-based cardiovascular and renal protective program. Medical Journal of Australia; 172: 473-478
- Menzies School of Health Research (1999) 1998-1999 Annual Report. Darwin: Menzies School of Health Research
- Hoy W, Vanbuynder P, Mathews J, Pugsley D, Wang Z (2001) Renal disease and the environment: lessons from Aboriginal Australia. Nephrology; 6: 19-24
- McDonald SP, Russ GR (2003) Current incidence, treatment patterns and outcome of end-stage renal disease among Indigenous groups in Australia and New Zealand. Nephrology; 8(1): 42-48
- Stewart JH, McCredie MRE, McDonald SP (2004) The incidence of treated end-stage renal disease in New Zealand Maori and Pacific Island people and in Indigenous Australians. Nephrology Dialysis Transplantation; 19(3): 678-685
- Stewart JH, McCredie MRE, McDonald SP (2004) Incidence of end-stage renal disease in overseas-born, compared with Australian-born, non-Indigenous Australians. Nephrology; 9(4): 247-252
- Cunningham J (2007) Making an IMPAKT: a national study aimed at improving access to optimal treatment for people with end-stage kidney disease. The Chronicle; 10(2): 17-18
- Anderson K, Cass A, Cunningham J, Snelling P, Devitt J, Preece C (2007) The use of psychosocial criteria in Australian patient selection guidelines for kidney transplantation. Social Science & Medicine; 64(10): 2107-2114
- Cunningham J, Cass A, Arnold PC (2005) Bridging the treatment gap for Indigenous Australians: demands for efficiency should not be met at the expense of equity [editorial]. Medical Journal of Australia; 182(10): 505-506
- NSW Health (2010) Kidney Health Check: promoting the early detection and management of chronic kidney disease. North Sydney: NSW Health
- Chadban S, Howell M, Twigg S, Thomas M, Jerums G, Alan C, Campbell D, Nicholls K, Tong A, Mangos G, Stack A, McIsaac R, Girgis S, Colagiuri R, Colagiuri S, Craig J (2009) National evidence based guideline for diagnosis, prevention and management of chronic kidney disease in type 2 diabetes. Canberra: National Health and Medical Research Council
- Hoy WE, Wang Z, Baker PRA, Kelly AM (2003) Secondary prevention of renal and cardiovascular disease: results of a renal and cardiovascular treatment program in an Australian Aboriginal community. Journal of the American Society of Nephrology; 14(supplement): 178-185
- National Health Priority Action Council (2006) National chronic disease strategy. Canberra: Australian Government Department of Health and Ageing
- Howard K, Salkeld G, White S, Chadban S, Craig J, McDonald S, Perkovic V, Cass A (2006) The cost-effectiveness of early detection and intervention to prevent the progression of chronic kidney disease in Australia. Melbourne: Kidney Health Australia
- Thomas MC (2007) Early detection of patients with kidney disease. Nephrology; 12(s1): S37
- Australian Institute of Health and Welfare (2009) Outline of the National Centre for Monitoring Chronic Kidney Disease. Canberra: Australian Institute of Health and Welfare
- The George Institute for Global Health (2011) Australian Department of Health and Ageing Central Australia Renal Study. Canberra: Australian Department of Health and Ageing
- Cass A, Chadban S, Craig J, Howard K, McDonald S, Salkeld G, White S (2006) The economic impact of end-stage kidney disease in Australia. Melbourne: Kidney Health Australia
- Cass A, Devitt J, Preece C, Cunningham J, Anderson K, Snelling P, Eris J, Ayanian J (2004) Barriers to access by Indigenous Australians to kidney transplantation: the IMPAKT study. Nephrology; 9(Supplement 4): s144-s146
- Coulehan K, Brown I, Christie M, Gorham G, Lowell A, Marrnanyin, Patel B (2005) Sharing the true stories: evaluating strategies to improve communication between health staff and Aboriginal patients: stage 2 report: September 2002 to August 2005. Darwin: Cooperative Research Centre for Aboriginal Health
- Human Rights and Equal Opportunity Commission (2008) Close the gap: Indigenous health equality summit, statement of intent. Canberra: Human Rights and Equal Opportunity Commission
- Council of Australian Governments (2009) National Indigenous reform agreement (closing the gap). Canberra: Council of Australian Governments
- COAG Reform Council (2011) National Indigenous Reform Agreement: performance report for 2009–10. Sydney: COAG Reform Council
- Council of Australian Governments (2009) National framework for protecting Australia’s children 2009–2020: protecting children is everyone’s business. Canberra: Council of Australian Governments
- Roxon N, Mclucas J (2009) Kidney disease – Australia’s ‘silent killer’. Retrieved 26 May 2009 from http://www.health.gov.au/internet/ministers/publishing.nsf/Content/1069A4C8CDC02D6ACA2575C2000AC3D0/$File/nr073.pdf
Acknowledgements
Special thanks are extended to:
- the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA) for the provision of the notification data on end-stage kidney disease (ESKD). (Interpretation and reporting of these data are the responsibility of the authors, however, and in no way should be seen as an official interpretation of ANZDATA.)
- Associate Professor Stephen McDonald for his expert guidance in the preparation of the review
- other staff of the Australian Indigenous HealthInfoNet, particularly to Leah Levitan, for their assistance, support and encouragement in the preparation of this review
- the Office for Aboriginal and Torres Strait Islander Health (OATSIH) within the Australian Department of Health and Ageing for their ongoing support of the work of the HealthInfoNet.
Endnotes
- The NATSIHS collected information from people living in private dwellings and did not include people in health care facilities, so may underestimate the prevalence of kidney disease in the Indigenous population.
- These data refer to episodes of admitted care, meaning the same patient can potentially have multiple hospitalisations within the same period. Consequently, data represents health service usage by those with CKD rather than representing the number or proportion of people in Australia with CKD admitted to hospital.
- Indigenous identification in deaths data is considered of sufficient quality for reporting only for NSW, Qld, WA, SA and the NT.
- The Index of Relative Advantage and Disadvantage is one of the ABS’s Socio-Economic Indexes For Areas (SEIFA) 2006, generated using a combination of 2006 Census data (i.e. income, education and dwelling size). Composite scores are indicative of the collective socioeconomic status of the people living in an area, with reference to the situation and standards applying to the wider community at a given point in time [68].
- Data do not represent the number or proportion of people in Australia with CKD admitted to hospital but rather health service usage by those with CKD.
- Due to data quality issues relating to Indigenous identification, hospital data by Indigenous status was restricted to hospitals in NSW, Vic, Qld, WA SA and public hospitals in the NT and hospitalisations where Indigenous status was missing or unknown were amalgamated with those identifying as non-Indigenous or ‘other’ Australians.
- The National Consumer Council of Kidney Health Australia advocates for the interests of kidney patients and carers throughout Australia. It comprises kidney patients and carers working together to improve the quality of life for people affected by kidney disease [128].



