Environmental health challenges in remote Aboriginal Australian communities: clean air, clean water and safe housing
ReviewHolly D. Clifford1*, Glenn Pearson1, Peter Franklin1,2, Roz Walker1, Graeme R. Zosky3
Environmental health challenges in remote Aboriginal Australian communities: clean air, clean water and safe housing. Australian Indigenous HealthBulletin 15(2). Retrieved [access date] from https://healthbulletin.org.au/articles/environmental-health-challenges-in-remote-aboriginal-australian-communities-clean-air-clean-water-and-safe-housing
1Telethon Kids Institute, University of Western Australia, Perth, Western Australia
2School of Paediatrics and Child Health, University of Western Australia, Perth, Western Australia
3School of Medicine, University of Tasmania, Hobart, Tasmania
*Corresponding author: Dr Holly D. Clifford, Telethon Kids Institute, 100 Roberts Road Subiaco, Western Australia 6008, ph: 61 8 9489 7787, fax: 61 8 9489 7700, email: holly.clifford@telethonkids.org.au
View PDF version (PDF – 1.1 MB)
Abstract
Objective: A considerable health disparity exists between Aboriginal and non-Aboriginal Australians, including a higher incidence and severity of cardiovascular and respiratory diseases. The burden of these diseases appears to be greatest in communities located in the remote regions of Australia. Unique environmental challenges in these regions may be a contributing factor; however these are yet to be adequately investigated. We aimed to develop a case to improve our understanding of environmental risk factors in remote Aboriginal communities.
Methods: We comprehensively reviewed the literature regarding physical environmental challenges that are likely to be highly prevalent in remote Aboriginal communities, and have been linked with adverse health. We focused on exposure to inhaled geogenic (earth-derived) dust and biomass smoke, bacterial and heavy metal contamination of drinking water and overcrowding.
Results: These environmental factors are anecdotally high in remote Aboriginal communities and have been linked, mostly epidemiologically, to cardiovascular, respiratory and other infectious diseases. These challenges are an under-recognised problem and are likely to have a significant impact on Aboriginal community health; increased research focus in this area would be of great benefit.
Implications: It is crucial to identify and quantify these physical environmental factors, and to determine the mechanisms through which they impact on health, particularly as these factors are modifiable and may be suppressed using relatively simple, cost-effective changes in community infrastructure. Protection against these exposures is likely to reduce their cumulative negative effects on individuals across the life course and result in significantly improved health in remote Aboriginal Australian communities.
Aboriginal and Torres Strait Islander Australians have a significant health disadvantage compared with other Australians, with a higher incidence and severity of many diseases including cardiovascular disease, respiratory diseases, renal disease and diabetes [1]. Mortality rates for Aboriginal Australians are almost twice as high as for non-Aboriginal individuals [2], and life expectancy is estimated to be up to 11.5 years less [3]. Furthermore, mortality rates of Aboriginal Australians are higher than those of other Indigenous populations elsewhere in the world [4]. There is an increasing impetus to identify the determining factors of this health disparity, with an overall aim of ‘closing the gap’ [5] and improving the health of Aboriginal Australians.
Previous studies have concentrated on the risk factors associated with poor health outcomes identified in developing countries, such as malnutrition, low birth weight, and tobacco smoke exposure [6]. There has been a particular focus on ‘choice-based’ lifestyle factors such as smoking and alcohol consumption, which are major causes of various health conditions including lung, cardiovascular and liver diseases [7,8]. Smoking and alcohol use in Aboriginal communities have a complex social aetiology associated with pervasive effects of colonisation on Aboriginal social, cultural and spiritual wellbeing, and represent a significant and ongoing challenge in Aboriginal health.
Outside of smoking and alcohol, many other factors may also account for the disease disparities with non-Aboriginal groups. There are many physical environmental factors that Aboriginal individuals may be uniquely exposed to which may have a significant impact on health. These include; geogenic (earth-derived) dust exposure, biomass smoke exposure, bacterial or heavy metal contamination of drinking water, and overcrowding. These are a selection of the environmental factors likely to be involved, with the scope of this review unable to exhaustively cover all contributing factors; other factors not discussed include house function and sewage and waste management, amongst others. The exposures reviewed herein are particularly relevant to remote Aboriginal communities where service delivery and infrastructure remain well below what is provided in urban and less remote centres, and are inadequate to support essential quality of life elements such as clean air, clean water and safe housing. Little consideration has been given to fully understanding the role some of these factors play in disease aetiology and severity in remote Aboriginal communities.
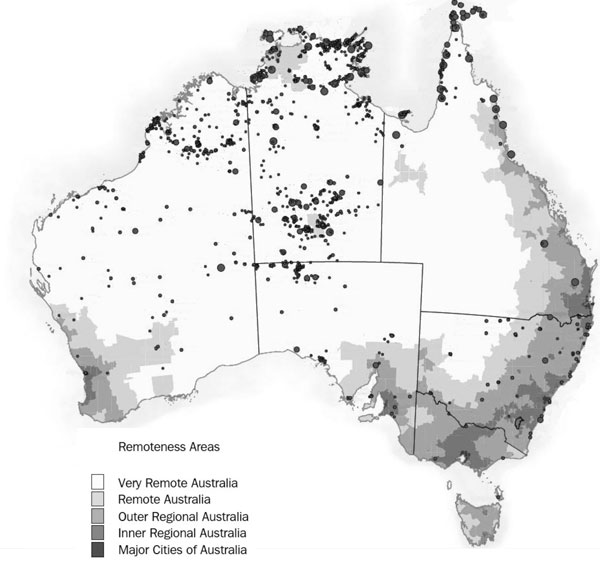
The Aboriginal population is more widely dispersed across Australia than the broader population [9], with discrete Aboriginal communities amply distributed across the remote areas of the country (Figure 1) [10]. Discrete Aboriginal communities range in size from less than 50 residents to greater than 1000 residents [11]. Approximately one quarter of Aboriginal people live in remote or very remote areas, compared with only 2% of the non-Aboriginal population [12]. Many of the health issues linked to alcohol and smoking in Aboriginal populations occur across urban, rural and remote areas. However, individuals in remote communities are likely to have additional challenges based on the unique geology, weather and environmental exposures found in the isolated areas of Australia.
Figure 1. Discrete Indigenous communities are widely dispersed across the remote and very remote regions of Australia
Note: Circles indicate relative size of community and shading from dark to light represents increasing remoteness
Source: Adapted from ABS 2006 [10]
Remoteness per se has been associated with adverse health outcomes within Aboriginal communities. The leading cause of Aboriginal mortality, cardiovascular disease, is more prevalent in Aboriginal communities in remote areas compared with urban areas [13]. Furthermore, children living in remote areas have higher hospitalisation rates for respiratory diseases, up to 3 times higher than those residing in metropolitan areas, even though they have less access to health services [14]. The reasons for these observations are not known but one potential explanation is the impact of the physical environment.
Unique physical environmental exposures in remote Aboriginal Australian communities have not been comprehensively quantified, and the mechanisms through which they cause adverse health effects have not been fully elucidated. As such, there are many unanswered questions in this field. It is not known whether physical environmental factors, and the levels of exposure, are unique to the remote regions of the country. Additionally, what is it about the physical environment that may be causing Aboriginal Australians to have such a high disease burden? This review will discuss a range of physical environmental factors which have been linked with poor health (Table 1), and are likely to be highly prevalent, and display unique characteristics, in remote Aboriginal Australian communities. This review draws on published studies and government reports to present what is known and also highlight what is not. The purpose of the review is to develop a case to improve our understanding of environmental risk factors that are often easily modifiable through simple investments in infrastructure.
| Physical environmental factor | Associations with health and disease | |||
|---|---|---|---|---|
| Inflammatory and disease biomarkers | Disease | Severity/Exacerbations | Mortality | |
Note:
|
||||
| Particulate air pollution | Increase in leukocytes in diabetics [15]Inflammatory mediators such as E-selectin [16]Pro-inflammatory cytokines such as IL-6 [17, 18]Cardiac biomarkers such as CRP and fibrinogen [17, 19-21]Inflammatory cell trafficking, oxidative stress and neuroinflammation [22] | Asthma and respiratory infections [6, 23-26]COPD and pulmonary fibrosis [27]Lung cancer [28]Cardiovascular diseases [29]Brain abnormalities and cognitive deficits [30] | Decreased lung function in cystic fibrosis patients [31]Asthma hospitalisations [32, 33]Cardiac arrest [34] Cardiovascular hospitalisations [35, 36]Stroke [37] | All cause [35, 38-43]Respiratory [40, 44]Cardiovascular [40, 42, 43, 45-47]Acute stroke [37, 48] |
| Mineral dusts | In vitro IL-6 and IL-8 cytokines [49] | Asbestosis and silicosis [50]Asthma [51]Parkinson’s-like neurodegeneration [52] | Asthma exacerbations [33, 53]Cardiovascular hospital admissions [54] | All cause [55]Respiratory [56-58]Lung cancer [57]Cardiovascular [57-59] |
| Biomass smoke | Pro-inflammatory mediators in vitro [60]Inflammatory cells [61-63]Cytokines such as IL-6, IL-8 [62] | Asthma [64, 65]COPD [66]Chronic bronchitis [67]Lung cancer [68]Congestive heart failure [65] | Respiratory hospital admissions [69-71]Asthma [71, 72]Acute lower respiratory tract infections [73-76]Ischaemic heart disease [77]Anaemia [73]Low birth weight [78-80] | All cause [55, 81, 82]Cardiovascular [55, 81, 82]Respiratory [81, 82]Infant mortality [78, 80] |
| Contamination of drinking water (bacteria or heavy metals) | Cytokines such as TNF-α [83]Cardiovascular markers such as VCAM-1 [84] | Diarrhoea and bacterial infection sequelae such as myocarditis and diabetes [85]Lower respiratory tract infections and diarrhoea [86]Bronchiectasis and obstructive lung diseases [87, 88]Cardiovascular diseases [89-91] | Impaired lung function [92-94]Anaemia [95]Cognitive decline [96] | All cause [97]Infectious diseases [97]Cancer [97, 98]Cardiovascular [98-101]Cerebrovascular disease, diabetes and kidney diseases [102] |
| Overcrowding | Acute lower respiratory illness [103]Bacterial carriage [104]Pneumonia [105, 106]Acute rheumatic fever [107]Meningococcal disease [108]Skin infections [109] | Acute hospitalisation rates [110] | Childhood mortality [111, 112] | |
Particulate air pollution
Ambient particulate air pollution in both cities and rural areas contributes to approximately 3.7 million premature deaths annually worldwide [113]. Particulate matter (PM) has been extensively linked epidemiologically to adverse health outcomes (Table 1). PM with a diameter of <10µm (PM10) is known as the inhalable fraction as it is small enough to reach, and hence damage, the lower respiratory tract [114]. Fine particles (PM2.5) can reach as far as the terminal alveoli [29] and ultrafine particles (PM0.1) can travel from the lung epithelium directly into the systemic circulation [115]. PM exposures have been associated with a wide range of respiratory diseases such as asthma and respiratory infections [6, 23-26], chronic obstructive pulmonary disease (COPD) and pulmonary fibrosis [27], and lung cancer [28] (Table 1). These exposures have also been linked with many cardiovascular diseases including hypertension and myocardial infarction [29], as well as disruption of the blood-brain barrier and brain abnormalities/damage [22, 30, 116]. Hospital admissions and mortality due to respiratory and cardiovascular exacerbations have also been extensively linked with PM [32, 33, 35, 36-40, 42, 43, 45-48] (Table 1). PM specifically from mineral dusts has also been associated with adverse health outcomes such as silicosis and fibrosis [50, 51, 56]. Recently, dust storms have been associated with increased hospital admissions due to COPD [54]. Dust inhalation may prime inflammatory cells in the lung to increase their capacity to release toxic oxygen radicals [117, 118] and initiate an inflammatory response [119-121] and this can lead to lung tissue damage [122] and, following travel into the systemic circulation, may also impact on cardiovascular outcomes [29]. Socio-economic disadvantage, existing chronic cardiovascular and respiratory disease, and diabetes have all been shown to modify the effect of particulate air pollution on health outcomes [123]. Aboriginal Australians have a high prevalence of these health risks and have been recognised as more likely to be at greater risk from poor air quality than other Australians [124].
Remote Aboriginal Australian communities are likely to be exposed to high levels of PM10. The national ambient air quality standard for PM10 in Australia is 50µg/m3 averaged over a 24-hour period, with ≤5 exceedances recommended within a year [125]. These standards are based mostly on international studies of urban particulates. However, in remote communities in arid regions of Australia, these air quality targets are regularly surpassed. For example, PM10 levels exceeded 50µg/m3 12 and 18 times in 2000 in Boodarie and Dampier, respectively [126]. Rather than the air pollution of major cities, which contains a large proportion of PM from vehicle emissions and carbonaceous sources, remote arid regions experience unique challenges. The physico-chemical features of PM10 from geogenic sources differ greatly depending on geographic location [127, 128] and airborne geogenic dusts are highly prevalent in remote areas due to their unique geology, dry climate, exposure to wind erosion and proximity to open cut mining activities [129].
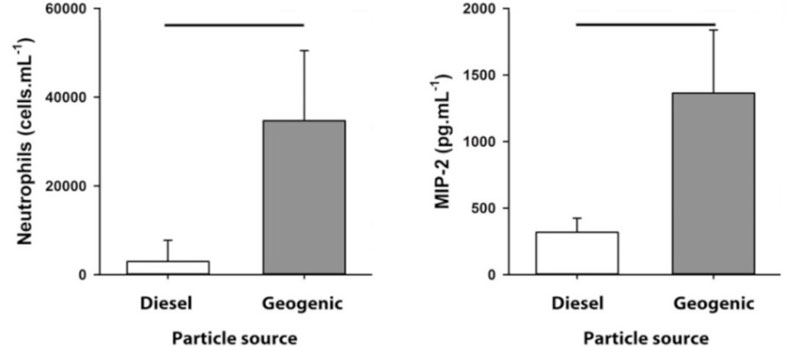
Urban particles have been shown to exacerbate the response to respiratory viral infection [130, 131], however the response to urban particles may be surpassed by the response to geogenic dust particles from remote areas. Geogenic PM10 sampled from remote Western Australian towns induced a greater inflammatory response compared with the same dose of diesel exhaust particulates (a major constituent of urban particles) [132] (Figure 2). How this affects the lung during respiratory infection or chronic lung disease is yet to be determined. The unique physico-chemical characteristics of geogenic PM10 may contribute to the observed differences with urban particles.
Figure 2. Geogenic particles from remote Western Australia induce a greater inflammatory response than urban diesel particles.
Note: Intranasal exposure to 30µg of geogenic particles from remote towns in Western Australia induced a greater influx of neutrophils and greater production of pro-inflammatory cytokine MIP-2 compared to the same dose of diesel exhaust particles in mice. Lines indicate p< 0.01.
Most studies on dust exposure have been in the occupational health setting, where a known, homogenous exposure (usually silica) is investigated in a selected population [133, 134], however these give a poor estimate of the impact of exposure on disease in the general population [135]. Based on animal data, chronic low level exposure to particles containing biologically active metals may be sufficient to impact on respiratory health at a community level. The properties of PM10 can determine the magnitude, duration and characteristics of the inflammatory response in the lung [127, 128, 136, 137]. Toxicological studies have shown that metals influence the toxicity of inhaled particles; metals such as lead and nickel can exert negative health effects from inhalation [128]. The nickel and vanadium content of PM2.5 was attributed to more than one-third of cardiovascular hospital admissions in the U.S. [47].
Iron content in geogenic PM10 may be particularly important. Carriage rates of several bacterial respiratory pathogens, including S. pneumoniae and H. influenzae, in the upper airway are particularly high in Aboriginal children [138]. Many bacterial species rely on iron acquisition during infection for their survival and virulence [139, 140]. Thus, iron-laden dust particle exposure, which is expected to be high in remote, arid regions, may be perpetuating chronic bacterial infections in Aboriginal children. This is supported by the fact that the rates of bacterial pneumonia in Aboriginal children were twice as high in the central arid regions of the Northern Territory compared to the northern tropical regions [141]. Additionally, a study comparing the lung health of residents in two remote W.A. Aboriginal communities found that residents from the arid community, compared with the tropical community, experienced more frequent respiratory symptoms and had poorer lung function – despite the lower smoking rates in the arid community [142]. The authors speculated that “environmental factors” may be playing a role in respiratory health in remote Aboriginal communities [142]. Furthermore, studies examining ‘red dust’ (iron ore) in Port Hedland, W.A. [129] and Whyalla, S.A. [143] showed that those exposed to higher levels had higher respiratory hospitalisation rates; specifically, districts living closest to iron ore stockpiles had higher rates of hospitalisation for acute respiratory illness than both the local and state average [129] and children 90% are in the inhalable size range [150]. Aboriginal Australian cooking methods originate in and around outdoor fires. Indoor biomass burning may be considered the major problem internationally [151], however, depending on proximity, exposure to outdoor biomass from cooking may still pose a risk to health, particularly when exposed chronically all through life.
Bushfires (or biomass fires) occur fairly regularly in Australia. The particularly arid climate and landscape of remote Aboriginal communities likely potentiates more frequent and severe bushfires. During the above average temperatures and exceptionally dry conditions associated with drought, the greatest areas that are burnt include Australia’s rangelands and northern savannas, where extensive bushfires particularly affect pastoralists and Aboriginal communities [152]. Additionally, Aboriginal Australians are involved in traditional burning practices for a number of reasons, including hunting and communication [153].
The pollutant most consistently elevated due to bushfire smoke is PM and during bushfire episodes PM concentrations several times above background urban concentrations can occur, with air quality standards commonly exceeded [154]. Bushfire smoke was found to have a major impact on air quality in regional N.S.W., where during bushfires, the air quality of impacted rural towns was almost twice that of the worst pollution levels in Sydney [155]. During a month-long prescribed burning season in Ovens, Victoria, smoke impacted the town for 12 days, of which 7 exceeded the PM2.5 air quality standard [155]. Furthermore, wildfires in this town caused the standard to be exceeded 13 times over 31 days [155].
Wood fire-derived PM is associated with a number of toxic co-pollutants including metals, organic and inorganic compounds [156]. In vitro studies have demonstrated that cellular responses depend on the chemical characteristics of the particles [157, 158]. Reports of the health impacts of wood smoke exposure range from irritation of the respiratory tract, through decreased lung function, to causal links with COPD, asthma and lung cancer, hospital visits and exacerbations, and finally, respiratory and cardiovascular related mortality [68, 151, 156, 159-163] (Table 1). Bushfire-specific PM10 has been associated with respiratory hospitalisations, particularly for COPD and asthma [71]. An increase of 10µg/m3 of PM10 was associated with a 5% increase in respiratory hospital admissions, with effect sizes being larger for Aboriginal people [77, 164]. The effects of PM10 from bushfires on respiratory and cardiovascular diseases are greater for Aboriginal compared with non-Aboriginal Australians [77].
Research needs
Bushfires are expected to increase in the future as a result of climate change [165]. The practice of deliberate landscape burning to avert major disasters has also increased but this has become increasingly controversial as the adverse health effects of PM air pollution become more widely known [152]. More research is required regarding the contribution of wood fires and bushfires to air quality in remote Aboriginal communities, as well as more information on the specific health impacts of these biomass particles. Although bushfires would be difficult to control in remote areas, awareness of the health impacts of biomass smoke PM may precipitate the implementation of effective strategies to reduce personal exposure.
Contamination of drinking water
The WHO estimates that about 1.1 billion people around the world are drinking unsafe water [166]. Furthermore, the majority (88%) of diarrhoeal disease in the world is attributable to unsafe water, sanitation and hygiene, as is approximately 3.1% (1.7 million) of all deaths annually [167]. Diarrhoea and related gastrointestinal (GI) illnesses continue to be one of the most important causes of morbidity and mortality, especially amongst young children [168]. A portion of this illness is due to exposure to contaminated water, as a result, for example, of poor water quality, limited access to water or hygiene practices. Examples of major pathogens that are found in contaminated water include Salmonella, Shigella, Campylobacter, E. coli and rotavirus.
Access to good quality drinking water that has acceptable levels of bacteria and heavy metals is an ongoing concern in remote Aboriginal communities. However, as with other environmental exposures discussed in this review, the health impacts of poor quality water in Aboriginal communities has not been fully addressed. Insufficient access to clean drinking water and functioning sewerage systems contributes to skin, eye and diarrhoeal diseases in Aboriginal communities [109, 169]. Gastroenteritis is second only to respiratory infections as the leading cause of hospitalisation for infection in children younger than 2 years, with rates up to 11 times higher in Aboriginal compared with non-Aboriginal children [170-173]. GI hospitalisation rates also vary between geographical locations, higher in remote versus non-remote regions [171-173]. Among Aboriginal children less than 5 years of age in W.A., the highest hospitalisation rates were found in the remote Kimberley and Pilbara-Gascoyne regions, where rates were 3.5 times higher than for Aboriginal children living in metropolitan areas [173].
Although jurisdictional differences do occur and some states have achieved compliance with Australian drinking water guidelines through regular testing and maintenance, for example in the N.T. [174], many areas in Australia still face issues with water quality and system management. In 2006, 978 discrete Aboriginal communities nationwide were not connected to the main town water supply. Of these communities, 17% had their drinking water sent away for testing, and 30% of these failed [11]. A further case in point is W.A., where almost half (49%) of the remote Aboriginal communities have untreated drinking water and 52% are without regular monthly testing of water quality [148].
Heavy metals are one of the most persistent water pollutants [175]. Degradation is difficult and therefore contamination accumulates in the environment. Metals are known to have adverse health impacts, even at low doses. A number of Australian towns have experienced water contamination issues. Shipping of lead (Pb) through Esperance Port in W.A. resulted in contamination and increased blood Pb concentrations in children [176, 177], and testing of rainwater tanks found concentrations of Pb and nickel (Ni) [175] exceeding the Australian drinking water guidelines [178, 179]. Lead is neurotoxic, and no threshold has been identified at which Pb exposure is safe to the developing nervous system [180]. Additionally, Ni has been associated with skin irritation and respiratory diseases such as asthma [181]. There have also been reports of heavy metal contamination of water in Tasmania, where one-third of Tasmania’s town water systems exceed the Australian drinking water guidelines for Pb, cadmium and arsenic [182]. The local drinking water source of the town of Royal George, an ex-tin mining town, was found to have arsenic levels 200 times the allowed standard [182]. High levels of arsenic exposure from drinking water have been related to elevated risks of, and mortality due to, cardiovascular diseases [100, 101], diabetes and kidney disease [102] (Table 1). In utero exposure to arsenic via the mother’s exposure to contaminated drinking water can impair somatic growth, lung development and lung mechanics [94] and it has therefore been linked to the higher incidence of bronchiectasis and obstructive lung diseases [87, 88].
Concerns have been raised regarding the contamination of groundwater with heavy metals in remote Aboriginal communities, particularly in communities where water is self-supplied and ongoing monitoring of the chemical content of the water is not conducted (more often the smaller communities) [183]. These concerns are also warranted because of the close proximity of many Aboriginal communities to mining activities. The Ranger mine in the N.T., for example, is leaking 100,000L of uranium-contaminated water into the groundwater beneath Kakadu every day [184]. Uranium is mined adjacent to traditional Aboriginal lands and ionising radiation from mining waste accumulates in Australian wildlife, some of which are traditional Aboriginal foods, such as mussels, turtles and fish [185]. There is an urgent requirement for ongoing monitoring of community drinking water sources and the provision of protection and prevention measures for Aboriginal communities living adjacent to mining operations.
Research needs
The degree of water contamination in remote Aboriginal communities must be quantified so as to reiterate the need for the regular screening and treatment of water sources in the remote regions of Australia. The treatment of water sources in remote communities is focussed on microbial contamination and primarily consists of UV and chlorine treatment. However, these are often not effective due to poor maintenance, and bacteria have often been detected in the water supply [145, 146]. Additionally, there is currently no mechanism for heavy metal remediation, even in those communities identified as being at risk due to mining activities [145]. This issue is further complicated by the substantial changes in runoff and underground water flow associated with the seasonal weather extremes experienced in northern Australia. Research on the specific effects of particular metals in contaminated water is also required, to determine the safe limits in order to set appropriate standards.
Overcrowding
A challenge that is highly prevalent in remote Aboriginal communities is based around housing and overcrowded living conditions. Overcrowding and its health implications can arise from the restricted availability of housing, inappropriate housing and community design, and poor housing conditions [186]. Overcrowding is one part of a greater issue of the provision of safe housing in remote Aboriginal communities. Overcrowding, according to the Australian Bureau of Statistics, takes into account household size and composition and is calculated based on the Canadian National Occupancy Standard [187]. When assessing bedroom requirements, households requiring at least one additional bedroom are considered to be overcrowded [187].
The average Aboriginal household is larger than a non-Aboriginal household [4]. Overcrowding is prevalent in Aboriginal Australians, with 26,000 (13%) Aboriginal households and 81,500 (25%) Aboriginal Australian adults living in overcrowded conditions [187]. In 2012-2013, 23% of Aboriginal Australians of all ages were living in overcrowded conditions [11]. Compared with their non-Aboriginal peers, Aboriginal adults were six times more likely to live in houses that required additional bedrooms [187]. This overcrowding is based on a family living in a house too small for their needs, or the cultural norm in which the household includes large numbers of extended family members, some of whom may have special needs (i.e. poor health from chronic conditions, recurrent infections). Furthermore, rates of overcrowding increases with remoteness, with only 17% of Aboriginal people of all ages in major cities experiencing overcrowding, while over half (53%) of Aboriginal people living in very remote areas are affected by overcrowded living conditions [11].
Overcrowding causes extra challenges in caring for children, as well as maintaining good personal and household hygiene [188]. Crowded living conditions and the consequent failure of health hardware due to increased use can lead to poor hygiene and may facilitate the spread of infectious diseases and the exacerbation of chronic conditions. A strong association between overcrowding and health exists even when factors including education, income, ethnicity, poverty and unemployment are taken into account [189]. Overcrowding puts increased stress on health infrastructure, such as water supply and sewage disposal systems, and is closely linked to housing standards and conditions. Overcrowding and poor housing quality has been linked with increased levels of life stressors, harmful alcohol consumption and social problems [190].
Health issues related to inadequate housing and infrastructure in remote regions of Australia include the spread of infectious diseases such as skin infections, respiratory infections, eye and ear infections, diarrhoeal diseases and rheumatic fever [103, 105, 106, 109, 191-193] (Table 1). These diseases are directly related to factors such as overcrowding, and Aboriginal children bear the greatest impact of these diseases [194]. In Aboriginal children, bacterial colonisation of the respiratory tract occurs soon after birth and their bacterial carriage rates are higher than those of non-Aboriginal children [195]. Overcrowding increases this risk of bacterial carriage, with the risk of carriage of S. pneumoniae, M. catarrhalis, and nontypeable H. influenzae increased with each additional household member in an overcrowded house [104].
Research needs
The N.S.W. Department of Health in partnership with the Department of Aboriginal Affairs has been delivering ‘Housing for Health’ projects in Aboriginal community housing sectors [196], undertaking the repair and maintenance of Aboriginal community housing with the specific intention of improving the safety and health of residents by addressing a variety of factors, including the impacts of overcrowding [196]. These projects have led to positive improvements in community health, including reduced rates of hospitalisations for infectious diseases (including respiratory, skin and intestinal infections, and otitis media), up to 40% lower than the rest of rural N.S.W. without the intervention [196]. Additionally, the Australian Government has committed to the ‘National Partnership Agreement on Remote Indigenous Housing’ which aims to build new homes and refurbish/rebuild existing homes to address the significant overcrowding and poor housing conditions in remote Aboriginal communities [197]. The establishment of high quality housing and related infrastructure, and the regulation of house crowding in remote Aboriginal communities, is vital to ensure more equitable health outcomes for Aboriginal Australians. Funding in the long term for these types of housing interventions, and the research demonstrating their effectiveness, is required.
Conclusions
It is important to identify the physical environmental challenges and their health implications in remote Aboriginal Australian communities. These factors may be easily modifiable and suppression of these exposures is likely to reduce their cumulative, negative effects on individuals across the life course and result in significant improvements in Aboriginal community health. The impact of environmental modifications needs to be properly assessed. At present, the exposure-disease associations are poorly characterised and difficult to quantify. Quantifying these associations is important for any health risk assessments and/or cost-benefit analyses of potential interventions. Systematic quantification of exposure levels in remote Aboriginal communities is needed, as is mechanistic evidence of how these exposures adversely affect health. Once a comprehensive evidence base is established, communities can lobby for a change in their living conditions that would provide protection from these preventable environmental exposures. Such interventions are strongly aligned with the ‘Closing the Gap targets’ [5].
Remediation practices should be of high priority in remote Aboriginal communities. These may include the sealing of roads and the establishment of re-vegetation programs in order to suppress exposure to dusts, or the review of ventilation standards for air-conditioning and housing (i.e. designing infrastructure for the environment) to limit geogenic or biomass PM10 exposure. This may also include water treatment systems or additional facilities so that there is an adequate water supply, and water quality, in these communities, or more effective housing/community design or housing interventions so as to reduce overcrowded households. These solutions can be expensive to implement, particularly in remote settings, and the efficacy of these solutions needs to be assessed. It is imperative that research in this area is translated into policy and practice so as to address these modifiable factors, and to have positive impacts on the health and wellbeing of Aboriginal Australians.
References
1. MacRae A, Thomson N, Anomie, Burns J, et al. Overview of Australian Indigenous health status, 2012. http://www.healthinfonet.ecu.edu.au/health-facts/overviews.
2. Australian Institute of Health and Welfare (AIHW). Life expectancy and mortality of Aboriginal and Torres Strait Islander people. Cat. no. IHW 51. 2011; Canberra: AIHW.
3. Australian Bureau of Statistics. Experimental life tables for Indigenous and Torres Strait Islander Australians: 2005-2007. 2009; Canberra: ABS.
4. Australian Bureau of Statistics. National Indigenous and Torres Strait Islander health survey, vol. (ABS Cat. No. 4715.0). 2005; Canberra: ABS.
5. Council of Australian Governments (COAG). National Aboriginal reform agreement (Closing the Gap). 2007; Canberra: COAG.
6. O’Grady KA, Chang AB. Lower respiratory infections in Australian Indigenous children. Journal of Paediatrics and Child Health 2010; 46(9): 461-465.
7. Australian Health Ministers’ Advisory Council. Aboriginal and Torres Strait Islander health performance framework: 2010 report. 2011; Canberra: Office for Aboriginal and Torres Strait Islander Health, Department of Health and Ageing.
8. Vos T, Barker B, Stanley L, Lopez A. The burden of disease and injury in Aboriginal and Torres Strait Islander peoples. 2003. 2007; Brisbane: Centre for Burden of Disease and Cost-Effectiveness, University of Queensland.
9. Australian Bureau of Statistics. Population characteristics, Indigenous and Torres Strait Islander Australians, 2006 (reissue). 2010; Canberra: ABS.
10. Australian Bureau of Statistics. Map of Australia – Discrete Indigenous communities and the Australian standard geographical classification remoteness structure (4706.0.30.001). maps and Census profiles, Australian Indigenous geographical classification. 2006; Canberra: ABS.
11. Steering Committee for the Review of Government Service Provision. Overcoming Indigenous disadvantage: key indicators. 2014; Canberra: Productivity Commission.
12. Department of Health and Aged Care. Measuring remoteness: accessibility/remoteness index of Australia (ARIA), revised edition. 2001; Canberra: Commonwealth Department of Health and Aged Care.
13. Australian Bureau of Statistics. National Indigenous and Torres Strait Islander health survey: Australia, 2004-05. 2006; Canberra: ABS.
14. Moore H, Burgner D, Carville K, Jacoby P, et al. Diverging trends for lower respiratory infections in non-Indigenous and Indigenous children. Journal of Paediatrics and Child Health 2007; 43(6): 451-457.
15. Jacobs L, Emmerechts J, Mathieu C, Hoylaerts MF, et al. Air pollution related prothrombotic changes in persons with diabetes. Environmental Health Perspectives 2010; 118(2): 191-196.
16. Hildebrandt K, Rückerl R, Koenig W, Schneider A, et al. Short-term effects of air pollution: a panel study of blood markers in patients with chronic pulmonary disease. Particle and Fibre Toxicology 2009; 6: 25.
17. Rückerl R, Greven S, Ljungman P, Aalto P, et al. Air pollution and inflammation (interleukin-6, C-reactive protein, fibrinogen) in myocardial infarction survivors. Environmental Health Perspectives 2007; 115(7): 1072-1080.
18. Tsai DH, Amyai N, Marques-Vidal P, Wang JL, et al. Effects of particulate matter on inflammatory markers in the general adult population. Particle and Fibre Toxicology 2012; 9: 24.
19. Rückerl R, Ibald-Mulli A, Koenig W, Schneider A, et al. Air pollution and markers of inflammation and coagulation in patients with coronary heart disease. American Journal of Respiratory and Critical Care Medicine 2006; 173(4): 432-441.
20. Wang G, Jiang R, Zhao Z, Song W. Effects of ozone and fine particulate matter (PM(2.5)) on rat system inflammation and cardiac function. Toxicology Letters 2013; 217(1): 23-33.
21. Khafaie MA, Salvi SS, Ojha A, Khafaie B, et al. Systemic inflammation (C-reactive protein) in type 2 diabetic patients is associated with ambient air pollution in pune city, India. Diabetes Care 2013; 36(3): 625-630.
22. Calderón-Garcidueñas L, Solt AC, Henríquez-Roldán C, Torres-Jardón R, et al. Long-term air pollution exposure is associated with neuroinflammation, an altered innate immune response, disruption of the blood-brain barrier, ultrafine particulate deposition, and accumulation of amyloid beta-42 and alpha-synuclein in children and young adults. Toxicological Pathology 2008; 36(2): 289-310.
23. Lin M, Stieb DM, Chen Y. Coarse particulate matter and hospitalization for respiratory infections in children younger than 15 years in Toronto: a case-crossover analysis. Pediatrics 2005; 116(2): e235-240.
24. Ko FW, Tam W, Wong TW, Lai CK, et al. Effects of air pollution on asthma hospitalization rates in different age groups in Hong Kong. Clinical and Experimental Allergy 2007; 37(9): 1312-1319.
25. Erbas B, Kelly AM, Physick B, Code C, et al. Air pollution and childhood asthma emergency hospital admissions: estimating intra-city regional variations. International Journal of Environmental Health Research 2005; 15(1): 11-20.
26. Canova C, Dunster C, Kelly FJ, Minelli C, et al. PM10-induced hospital admissions for asthma and chronic obstructive pulmonary disease: The modifying effect of individual characteristics. Epidemiology 2012; 23(4): 607-615.
27. Vincent JH. Health-related aerosol measurement: a review of existing sampling criteria and proposals for new ones. Journal of Environmental Monitoring 2005; 7(11): 1037-1053.
28. Pope CA 3rd, Burnett RT, Thun MJ, Calle EE, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. Journal of the American Medical Association 2002; 287(9): 1132-1141.
29. Nogueira JB. Air pollution and cardiovascular disease. Revista Portuguesa de Cardiologia 2009; 28(6): 715-733.
30. Calderón-Garcidueñas L, Mora-Tiscareño A, Ontiveros E, Gómez-Garza G, et al. Air pollution, cognitive deficits and brain abnormalities: a pilot study with children and dogs. Brain and Cognition 2008; 68(2): 117-127.
31. Goss CH, Newsom SA, Schildcrout JS, Sheppard L, et al. Effect of ambient air pollution on pulmonary exacerbations and lung function in cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2004; 169(7): 816-821.
32. Andersen ZJ, Bønnelykke K, Hvidberg M, Jensen SS, et al. Long-term exposure to air pollution and asthma hospitalisations in older adults: a cohort study. Thorax 2012; 67(1): 6-11.
33. Samoli E, Nastos PT, Paliatsos AG, Katsouyanni K, et al. Acute effects of air pollution on pediatric asthma exacerbation: evidence of association and effect modification. Environmental Research 2011; 111(3): 418-424.
34. Dennekamp M, Akram M, Abramson MJ, Tonkin A, et al. Outdoor air pollution as a trigger for out-of-hospital cardiac arrests. Epidemiology 2010; 21(4): 494-500.
35. Pope CA 3rd, Dockery DW. Health effects of fine particulate air pollution: lines that connect. Journal of the Air and Waste Management Association 2006; 56(6): 709-742.
36. Peng RD, Chang HH, Bell ML, McDermott A, et al. Coarse particulate matter air pollution and hospital admissions for cardiovascular and respiratory diseases among Medicare patients. Journal of the American Medical Association 2008; 299(18): 2172-2179.
37. Maheswaran R, Pearson T, Campbell MJ, Haining RP, et al. A protocol for investigation of the effects of outdoor air pollution on stroke incidence, phenotypes and survival using the South London Stroke Register. International Journal of Health and Geography 2006; 5: 10.
38. Samet JM, Dominici F, Curriero FC, Coursac I, et al. Fine particulate air pollution and mortality in 20 U.S. cities, 1987-1994. New England Journal of Medicine 2000; 343(24): 1742-1749.
39. Brook RD, Rajagopalan S. Particulate matter air pollution and atherosclerosis. Current Atherosclerosis Reports 2010; 12(5): 291-300.
40. Samoli E, Analitis A, Touloumi G, Schwartz J, et al. Estimating the exposure-response relationships between particulate matter and mortality within the APHEA multicity project. Environmental Health Perspectives 2005; 113(1): 88-95.
41. Breitner S, Stölzel M, Cyrys J, Pitz M, et al. Short-term mortality rates during a decade of improved air quality in Erfurt, Germany. Environmental Health Perspectives 2009; 117(3): 448-454.
42. Wichmann HE, Spix C, Tuch T, Wölke G, et al. Daily mortality and fine and ultrafine particles in Erfurt, Germany part I: role of particle number and particle mass. Research Report/ Health Effects Institute 2000; (98): 5-86.
43. Stölzel M, Breitner S, Cyrys J, Pitz M, et al. Daily mortality and particulate matter in different size classes in Erfurt, Germany. Journal of Exposure Science and Environmental Epidemiology 2007; 17(5): 458-467.
44. Viegi G, Scognamiglio A, Baldacci S, Pistelli F, et al. Epidemiology of chronic obstructive pulmonary disease (COPD). Respiration 2001; 68(1): 4-19.
45. Forastiere F, Stafoggia M, Picciotto S, Bellander T, et al. A case-crossover analysis of out-of-hospital coronary deaths and air pollution in Rome, Italy. American Journal of Respiratory and Critical Care Medicine 2005; 172(12): 1549-1555.
46. Kettunen J, Lanki T, Tiittanen P, Aalto PP, et al. Associations of fine and ultrafine particulate air pollution with stroke mortality in an area of low air pollution levels. Stroke 2007; 38(3): 918-922.
47. Bell ML, Ebisu K, Peng RD, Samet JM, et al. Hospital admissions and chemical composition of fine particle air pollution. American Journal of Respiratory and Critical Care Medicine 2009; 179(12): 1115-1120.
48. Hong YC, Lee JT, Kim H, Ha EH, et al. Effects of air pollutants on acute stroke mortality. Environmental Health Perspectives 2002; 110(2): 187-191.
49. Veranth JM, Moss TA, Chow JC, Labban R, et al. Correlation of in vitro cytokine responses with the chemical composition of soil-derived particulate matter. Environmental Health Perspectives 2006; 114(3): 341-349.
50. Mossman BT, Churg A. Mechanisms in the pathogenesis of asbestosis and silicosis. American Journal of Respiratory and Critical Care Medicine 1998; 157(5 Pt 1): 1666-80.
51. Eagan TM, Gulsvik A, Eide GE, Bakke PS. Occupational airborne exposure and the incidence of respiratory symptoms and asthma. American Journal of Respiratory and Critical Care Medicine 2002; 166(7): 933-938.
52. Lucchini R, Albini E, Benedetti L, Zoni S, et al. Neurological and neuropsychological features in Parkinsonian patients exposed to neurotoxic metals. Italian Journal of Occupational Medicine and Ergonomics 2007; 29(3 Suppl): 280-281.
53. Kanatani KT, Ito I, Al-Delaimy WK, Adachi Y, et al. Desert dust exposure is associated with increased risk of asthma hospitalization in children. American Journal of Respiratory and Critical Care Medicine 2010; 182(12): 1475-1481.
54. Tam WW, Wong TW, Wong AH, Hui DS. Effect of dust storm events on daily emergency admissions for respiratory diseases. Respirology 2012; 17(1): 143-148.
55. Johnston F, Hanigan I, Henderson S, Morgan G, et al. Extreme air pollution events from bushfires and dust storms and their association with mortality in Sydney, Australia 1994-2007. Environmental Research 2011; 111(6): 811-816.
56. Matheson MC, Benke G, Raven J, Sim MR, et al. Biological dust exposure in the workplace is a risk factor for chronic obstructive pulmonary disease. Thorax 2005; 60(8): 645-651.
57. Chen W, Liu Y, Wang H, Hnizdo E, et al. Long-term exposure to silica dust and risk of total and cause-specific mortality in Chinese workers: a cohort study. PLoS Medicine 2012; 9(4): e1001206.
58. Kashima S, Yorifuji T, Tsuda T, Eboshida A. Asian dust and daily all-cause or cause-specific mortality in western Japan. Occupational and Environmental Medicine 2012; 69(12): 908-915.
59. Torén K, Bergdahl IA, Nilsson T, Järvholm B. Occupational exposure to particulate air pollution and mortality due to ischaemic heart disease and cerebrovascular disease. Occupational and Environmental Medicine 2007; 64(8): 515-519.
60. Bølling AK, Totlandsdal AI, Sallsten G, Braun A, et al. Wood smoke particles from different combustion phases induce similar pro-inflammatory effects in a co-culture of monocyte and pneumocyte cell lines. Particle and Fibre Toxicology 2012; 9: 45.
61. Tan WC, Qiu D, Liam BL, Ng TP, et al. The human bone marrow response to acute air pollution caused by forest fires. American Journal of Respiratory and Critical Care Medicine 2000; 161(4 Pt 1): 1213-1217.
62. Swiston JR, Davidson W, Attridge S, Li GT, et al. Wood smoke exposure induces a pulmonary and systemic inflammatory response in firefighters. European Respiratory Journal 2008; 32(1): 129-138.
63. Mondal NK, Das D, Mukherjee B, Ray MR. Upregulation of AgNOR expression in epithelial cells and neutrophils in the airways and leukocytes in peripheral blood of women chronically exposed to biomass smoke. Analytical and Quantitative Cytology and Histology 2011; 33(1): 50-59.
64. Schei MA, Hessen JO, Smith KR, Bruce N, et al. Childhood asthma and indoor woodsmoke from cooking in Guatemala. Journal of Exposure Analysis and Environmental Epidemiology 2004; 14 (Suppl 1): S110-117.
65. Rappold AG, Cascio WE, Kilaru VJ, Stone SL, et al. Cardio-respiratory outcomes associated with exposure to wildfire smoke are modified by measures of community health. Environmental Health 2012; 11: 71.
66. Caballero A, Torres-Duque CA, Jaramillo C, Bolívar F, et al. Prevalence of COPD in five Colombian cities situated at low, medium, and high altitude (PREPOCOL study). Chest 2008; 133(2): 343-349.
67. Pérez-Padilla R, Regalado J, Vedal S, Paré P, et al. Exposure to biomass smoke and chronic airway disease in Mexican women. A case-control study. American Journal of Respiratory and Critical Care Medicine 1996; 154(3 Pt 1): 701-706.
68. Behera D, Balamugesh T. Indoor air pollution as a risk factor for lung cancer in women. Journal of the Association of Physicians India 2005; 53: 190-192.
69. Chen L, Verrall K, Tong S. Air particulate pollution due to bushfires and respiratory hospital admissions in Brisbane, Australia. International Journal of Environmental Health Research 2006; 16(3): 181-191.
70. Tham R, Erbas B, Akram M, Dennekamp M, et al. The impact of smoke on respiratory hospital outcomes during the 2002-2003 bushfire season, Victoria, Australia. Respirology 2009; 14(1): 69-75.
71. Morgan G, Sheppeard V, Khalaj B, Ayyar A, et al. Effects of bushfire smoke on daily mortality and hospital admissions in Sydney, Australia. Epidemiology 2010; 21(1): 47-55.
72. Chew FT, Ooi BC, Hui JK, Saharom R, et al. Singapore’s haze and acute asthma in children. Lancet 1995; 346(8987): 1427.
73. Mishra V, Retherford RD. Does biofuel smoke contribute to anaemia and stunting in early childhood? International Journal of Epidemiology 2007; 36(1): 117-129.
74. Smith KR, Samet JM, Romieu I, Bruce N. Indoor air pollution in developing countries and acute lower respiratory infections in children. Thorax 2000; 55(6): 518-532.
75. Viegi G, Simoni M, Scognamiglio A, Baldacci S, et al. Indoor air pollution and airway disease. International Journal of Tuberculosis and Lung Diseases 2004; 8(12): 1401-1415.
76. Po JY, FitzGerald JM, Carlsten C. Respiratory disease associated with solid biomass fuel exposure in rural women and children: systematic review and meta-analysis. Thorax 2011; 66(3): 232-239.
77. Johnston FH, Bailie RS, Pilotto LS, Hanigan IC. Ambient biomass smoke and cardio-respiratory hospital admissions in Darwin, Australia. BMC Public Health 2007; 7: 240.
78. Rinne ST, Rodas EJ, Rinne ML, Simpson JM, et al. Use of biomass fuel is associated with infant mortality and child health in trend analysis. American Journal of Tropical Medicine and Hygiene 2007; 76(3): 585-591.
79. Sreeramareddy CT, Shidhaye RR, Sathiakumar N. Association between biomass fuel use and maternal report of child size at birth–an analysis of 2005-06 India Demographic Health Survey data. BMC Public Health 2011; 11: 403.
80. Tielsch JM, Katz J, Thulasiraj RD, Coles CL, et al. Exposure to indoor biomass fuel and tobacco smoke and risk of adverse reproductive outcomes, mortality, respiratory morbidity and growth among newborn infants in south India. International Journal of Epidemiology 2009; 38(5): 1351-1363.
81. Analitis A, Georgiadis I, Katsouyanni K. Forest fires are associated with elevated mortality in a dense urban setting. Occupational and Environmental Medicine 2012; 69(3): 158-162.
82. Johnston FH, Hanigan IC, Henderson SB, Morgan GG. Evaluation of interventions to reduce air pollution from biomass smoke on mortality in Launceston, Australia: retrospective analysis of daily mortality, 1994-2007. British Medical Journal 2013; 346: e8446.
83. Ahmed S, Mahabbat-e Khoda S, Rekha RS, Gardner RM, et al. Arsenic-associated oxidative stress, inflammation, and immune disruption in human placenta and cord blood. Environmental Health Perspectives 2011; 119(2): 258-264.
84. Wu F, Jasmine F, Kibriya MG, Liu M, et al. Association between arsenic exposure from drinking water and plasma levels of cardiovascular markers. American Journal of Epidemiology 2012; 175(12): 1252-1261.
85. Ashbolt NJ. Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology 2004; 198(1-3): 229-238.
86. Rahman A, Vahter M, Ekström EC, Persson LÅ. Arsenic exposure in pregnancy increases the risk of lower respiratory tract infection and diarrhea during infancy in Bangladesh. Environmental Health Perspectives 2011; 119(5): 719-724.
87. De B, Majumdar D, Sen S, Guru S, et al. Pulmonary involvement in chronic arsenic poisoning from drinking contaminated ground-water. Journal of the Association of Physicians India 2004; 52: 395-400.
88. Mazumder DN, Steinmaus C, Bhattacharya P, von Ehrenstein O, et al. Bronchiectasis in persons with skin lesions resulting from arsenic in drinking water. Epidemiology 2005, 16(6): 760-765.
89. Chen CJ, Hsueh YM, Lai MS, Shyu MP, et al. Increased prevalence of hypertension and long-term arsenic exposure. Hypertension 1995; 25(1): 53-60.
90. Chiou HY, Huang WI, Su CL, Chang SF, et al. Dose-response relationship between prevalence of cerebrovascular disease and ingested inorganic arsenic. Stroke 1997; 28(9): 1717-1723.
91. Wang CH, Jeng JS, Yip PK, Chen CL, et al. Biological gradient between long-term arsenic exposure and carotid atherosclerosis. Circulation 2002; 105(15): 1804-1809.
92. Chattopadhyay BP, Mukherjee AK, Gangopadhyay PK, Alam J, et al. Respiratory effect related to exposure of different concentrations of arsenic in drinking water in West Bengal, India. Journal of Environmental Science and Engineering 2010; 52(2): 147-154.
93. Liao CM, Chio CP, Cheng YH, Hsieh NH, et al. Quantitative links between arsenic exposure and influenza A (H1N1) infection-associated lung function exacerbations risk. Risk Analysis 2011; 31(8): 1281-1294.
94. Ramsey KA, Larcombe AN, Sly PD, Zosky GR. In utero exposure to low dose arsenic via drinking water impairs early life lung mechanics in mice. BMC Pharmacology and Toxicology 2013; 14(1): 13.
95. Merrill RD, Shamim AA, Ali H, Labrique AB, et al. High prevalence of anemia with lack of iron deficiency among women in rural Bangladesh: a role for thalassemia and iron in groundwater. Asia Pacific Journal of Clinical Nutrition 2012; 21(3): 416-424.
96. Solfrizzi V, Colacicco AM, D’Introno A, Capurso C, et al. Macronutrients, aluminium from drinking water and foods, and other metals in cognitive decline and dementia. Journal of Alzheimers Disease 2006; 10(2-3): 303-330.
97. Sohel N, Persson LA, Rahman M, Streatfield PK, et al. Arsenic in drinking water and adult mortality: a population-based cohort study in rural Bangladesh. Epidemiology 2009; 20(6): 824-830.
98. Lewis DR, Southwick JW, Ouellet-Hellstrom R, Rench J, et al. Drinking water arsenic in Utah: A cohort mortality study. Environmental Health Perspectives 1999; 107(5): 359-365.
99. Medrano MA, Boix R, Pastor-Barriuso R, Palau M, et al. Arsenic in public water supplies and cardiovascular mortality in Spain. Environmental Research 2010; 110(5): 448-454.
100. Chen CJ, Chiou HY, Chiang MH, Lin LJ, et al. Dose-response relationship between ischemic heart disease mortality and long-term arsenic exposure. Arteriosclerosis, Thrombosis, and Vascular Biology 1996; 16(4): 504-510.
101. Tseng CH, Chong CK, Tseng CP, Hsueh YM, et al. Long-term arsenic exposure and ischemic heart disease in arseniasis-hyperendemic villages in Taiwan. Toxicology Letters 2003; 137(1-2): 15-21.
102. Meliker JR, Wahl RL, Cameron LL, Nriagu JO. Arsenic in drinking water and cerebrovascular disease, diabetes mellitus, and kidney disease in Michigan: a standardized mortality ratio analysis. Environmental Health 2007; 6: 4.
103. Prietsch SO, Fischer GB, César JA, Lempek BS, et al. Acute lower respiratory illness in under-five children in Rio Grande, Rio Grande do Sul State, Brazil: prevalence and risk factors. Cadernos Saude Publica 2008; 24(6): 1429-1438.
104. Jacoby P, Carville KS, Hall G, Riley TV, et al. Crowding and other strong predictors of upper respiratory tract carriage of otitis media-related bacteria in Australian Aboriginal and non-Aboriginal children. Pediatric Infectious Disease Journal 2011; 30(6): 480-485.
105. Hasan K, Jolly P, Marquis G, Roy E, et al. Viral etiology of pneumonia in a cohort of newborns till 24 months of age in Rural Mirzapur, Bangladesh. Scandinavian Journal of Infectious Disease 2006; 38(8): 690-695.
106. Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, et al. Epidemiology and etiology of childhood pneumonia. Bulletin of the World Health Organization 2008; 86(5): 408-416.
107. Jaine R, Baker M, Venugopal K. Acute rheumatic fever associated with household crowding in a developed country. Pediatric Infectious Disease Journal 2011; 30(4): 315-319.
108. Baker M, McNicholas A, Garrett N, Jones N, et al. Household crowding a major risk factor for epidemic meningococcal disease in Auckland children. Pediatric Infectious Disease Journal 2000; 19(10): 983-990.
109. Bailie RS, Stevens MR, McDonald E, Halpin S, et al. Skin infection, housing and social circumstances in children living in remote Indigenous communities: testing conceptual and methodological approaches. BMC Public Health 2005; 5: 128.
110. Jackson G, Thornley S, Woolston J, Papa D, et al. Reduced acute hospitalisation with the healthy housing programme. Journal of Epidemiology and Community Health 2011; 65(7): 588-593.
111. Coggon D, Barker DJ, Inskip H, Wield G. Housing in early life and later mortality. Journal of Epidemiology and Community Health 1993; 47(5): 345-348.
112. Kuate Defo B. Determinants of infant and early childhood mortality in Cameroon: the role of socioeconomic factors, housing characteristics, and immunization status. Society of Biology 1994; 41(3-4): 181-211.
113. World Health Organization (WHO). Ambient (outdoor) air quality and health. Fact Sheet No 313. World Health Organization, Geneva. 2014.
114. Olivieri D, Scoditti E. Impact of environmental factors on lung defences. European Respiratory Reviews 2005; 14(95): 51-56.
115. Geiser M. Morphological aspects of particle uptake by lung phagocytes. Microscopy Research and Technique 2002; 57(6): 512-522.
116. Calderón-Garcidueñas L, Reed W, Maronpot RR, Henríquez-Roldán C, et al. Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicologic Pathology 2004; 32(6): 650-658.
117. Cantin A, Dubois F, Bégin R. Lung exposure to mineral dusts enhances the capacity of lung inflammatory cells to release superoxide. Journal of Leukocyte Biology 1988; 43(4): 299-303.
118. Doelman CJ, Leurs R, Oosterom WC, Bast A. Mineral dust exposure and free radical-mediated lung damage. Experimental Lung Research 1990; 16(1): 41-55.
119. Driscoll KE, Hassenbein DG, Carter J, Poynter J, et al. Macrophage inflammatory proteins 1 and 2: expression by rat alveolar macrophages, fibroblasts, and epithelial cells and in rat lung after mineral dust exposure. American Journal of Respiratory Cell and Molecular Biology 1993; 8(3): 311-318.
120. Driscoll KE, Howard BW, Carter JM, Asquith T, et al. Alpha-quartz-induced chemokine expression by rat lung epithelial cells: effects of in vivo and in vitro particle exposure. American Journal of Pathology 1996; 149(5): 1627-1637.
121. Rimal B, Greenberg AK, Rom WN. Basic pathogenetic mechanisms in silicosis: current understanding. Current Opinion in Pulmonary Medicine 2005; 11(2): 169-173.
122. Yu M, Zheng X, Witschi H, Pinkerton KE. The role of interleukin-6 in pulmonary inflammation and injury induced by exposure to environmental air pollutants. Toxicological Sciences 2002; 68(2): 488-497.
123. O’Neill MS, Jerrett M, Kawachi L, Levy JL, et al. Health, wealth, and air pollution: advancing theory and methods. Environmental Health Perspectives 2003; 111(16): 1861-1870.
124. Trewin D. National Aboriginal and Torres Strait Islander health survey, 2004–05. Cat. No. 4715.0. 2006; Canberra: ABS.
125. Department of the Environment. National standard for criteria air pollutants in Australia. 2005. www.environment.gov.au/atmosphere/airquality/publications/standards.html# fn1.
126. W.A. Department of Environmental Protection. Monitoring of ambient air quality and meteorology during the Pilbara air quality study. 2002.
127. Zosky GR, Boylen CE, Wong RS, Smirk MN, et al. Variability and consistency in lung inflammatory responses to particles with a geogenic origin. Respirology 2014; 19(1): 58-66.
128. Harrison RM, Yin J. Particulate matter in the atmosphere: which particle properties are important for its effects on health? Science of the Total Environment 2000; 249(1-3): 85-101.
129. Mullan N, Codde J, Van Buynder P. Respiratory hospitalisations in Port Hedland, 1993-2004: An exploratory geographical analysis. Epidemiology Branch W.A. Department of Health Report. 2006.
130. Larcombe AN, Foong RE, Boylen CE, Zosky GR. Acute diesel exhaust particle exposure increases viral titre and inflammation associated with existing influenza infection, but does not exacerbate deficits in lung function. Influenza and Other Respiratory Viruses 2013; 7(5): 701-709.
131. Lambert AL, Mangum JB, DeLorme MP, Everitt JI. Ultrafine carbon black particles enhance respiratory syncytial virus-induced airway reactivity, pulmonary inflammation, and chemokine expression. Toxicological Sciences 2003; 72(2): 339-346.
132. Boylen CE, Sly PD, Zosky GR, Larcombe AN. Physiological and inflammatory responses in an anthropomorphically relevant model of acute diesel exhaust particle exposure are sex and dose-dependent. Inhalation Toxicology 2011; 23(14): 906-917.
133. Hnizdo E, Vallyathan V. Chronic obstructive pulmonary disease due to occupational exposure to silica dust: A review of epidemiological and pathological evidence. Occupational and Environmental Medicine 2003; 60: 237-243.
134. Vallyathan V, Shi X, Dalal NS, Irr W, et al. Generation of free radicals from freshly fractured silica dust: Potential role in acute silica-induced lung injury. American Journal of Respiratory and Critical Care Medicine 1988; 138(5): 1213-1219.
135. Baillargeon J. Characteristics of the healthy worker effect. Occupational Medicine 2001; 16(2): 359-366.
136. Schins RP. Mechanisms of genotoxicity of particles and fibers. Inhalation Toxicology 2002; 14(1): 57-78.
137. Cook AG, Weinstein P, Centeno JA. Health effects of natural dust: role of trace elements and compounds. Biological Trace Element Research 2005; 103(1): 1-15.
138. Watson K, Carville K, Bowman J, Jacoby P, et al. Upper respiratory tract bacterial carriage in Aboriginal and non-Aboriginal children in a semi-arid area of Western Australia. Pediatric Infectious Disease Journal 2006; 25(9): 782-790.
139. Somerville GA, Proctor RA. At the crossroads of bacterial metabolism and virulence factor synthesis in staphylococci. Microbiology and Molecular Biology Reviews 2009; 73: 233-248.
140. Skaar EP. The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathogens 2010; 6: e1000949.
141. O’Grady KA, Taylor-Thomson DM, Chang AB, Torzillo PJ, et al. Rates of radiologically confirmed pneumonia as defined by the World Health Organization in Northern Territory Indigenous children. Medical Journal of Australia 2010; 192(10): 592-595.
142. Verheijden MW, Ton A, James AL, Wood M, et al. Respiratory morbidity and lung function in two Aboriginal communities in Western Australia. Respirology 2002; 7(3): 247-253.
143. South Australian Department of Health. Whyalla health impact study report. 2007.
144. Zosky GR, Iosifidis T, Perks K, Ditcham WG, Devadason SG, Siah WS, Devine B, Maley F, Cook A. The concentration of iron in real-world geogenic PM10 is associated with increased inflammation and deficits in lung function in mice. PLoS One 2014; 9(2): e90609.
145. Western Australian Planning Commission. Parnngurr (Cotton Creek) community layout plan report and provisions. 2007. www.planning.wa.gov.au
146. Western Australian Planning Commission. Punmu (Lake Dora) Community Layout plan, report and provisions. 2007. www.planning.wa.gov.au
147. Nganampa Health Council. Report of Uwankara Palyanyku Kanyintjaku: An environmental and public health review within Anangu Pitjantjatjara lands. 1987.
148. Environmental Health Needs Coordinating Committee (EHNCC). Environmental health needs of Aboriginal communities in Western Australia. The 2008 survey and its findings. 2008. http://www.public.health.wa.gov.au/2/121/1/reports.pm
149. World Resources Institute, UNEP, UNDP, World Bank. 1998-1999 world resources: a guide to global environment. Oxford University Press 1998.
150. Torres-Duque C, Maldonado D, Perez-Padilla R, Ezzati M, et al. Forum of International Respiratory Studies (FIRS) Task Force on Health Effects of Biomass Exposure. Biomass fuels and respiratory diseases: a review of the evidence. Proceedings of the American Thoracic Society 2008; 5(5): 577–590.
151. Kodgule R, Salvi S. Exposure to biomass smoke as a cause for airway disease in women and children. Current Opinion in Allergy and Clinical Immunology 2012; 12(1): 82-90.
152. Ellis S, Kanowski P, Whelan R. National Inquiry on Bushfire Mitigation and Management. 2004; Canberra: Commonwealth of Australia.
153. Department of Land Resource Management. Aboriginal burning practices. Bushfires NT. 2013. http://www.lrm.nt.gov.au/bushfires/aboriginal.
154. Reisen F, Brown SK. Australian firefighters’ exposure to air toxics during bushfire burns of autumn 2005 and 2006. Environment International 2009; 35(2): 342-352.
155. Meyer CP, Reisen F, Luhar A, Powell J, et al. Particles, ozone and air toxic levels in rural communities during prescribed burning seasons. CSIRO, 2008. http://www.environment.gov.au/system/files/resources/ca57f9b8-1987-4800-9254-2acb3d21 9bfa/files/particles-ozone-toxic.pdf.
156. Naeher LP, Brauer M, Lipsett M, Zelikoff JT, et al. Woodsmoke health effects: a review. Inhalation Toxicology 2007; 19(1): 67-106.
157. Aust AE, Ball JC, Hu AA, Lighty JS, et al. Particle characteristics responsible for effects on human lung epithelial cells. Research report (Health Effects Institute) 2002; (110): 1-65.
158. Schwarze PE, Øvrevik J, Hetland RB, Becher R, et al. Importance of size and composition of particles for effects on cells in vitro. Inhalation Toxicology 2007; 19 (Suppl 1): 17-22.
159. Sandoval J, Salas J, Martinez-Guerra ML, Gómez A, et al. Pulmonary arterial hypertension and cor pulmonale associated with chronic domestic woodsmoke inhalation. Chest 1993; 103(1): 12-20.
160. Larson TV, Koenig JQ. Wood smoke: emissions and noncancer respiratory effects. Annual Review of Public Health 1994; 15: 133-156.
161. Peel JL, Tolbert PE, Klein M, Metzger KB, et al. Ambient air pollution and respiratory emergency department visits. Epidemiology 2005; 16(2): 164-174.
162. Barregard L, Sällsten G, Gustafson P, Andersson L, et al. Experimental exposure to wood-smoke particles in healthy humans: effects on markers of inflammation, coagulation, and lipid peroxidation. Inhalation Toxicology 2006; 18(11): 845-853.
163. Orozco-Levi M, Garcia-Aymerich J, Villar J, Ramírez-Sarmiento A, et al. Wood smoke exposure and risk of chronic obstructive pulmonary disease. European Respiratory Journal 2006; 27(3): 542-546.
164. Hanigan IC, Johnston FH, Morgan GG. Vegetation fire smoke, indigenous status and cardio-respiratory hospital admissions in Darwin, Australia, 1996-2005: a time-series study. Environmental Health 2008; 7: 42.
165. Schwela D. Fire disasters: The WHO-UNEP-WMO health guidelines for vegetation fire events. Annals of Burns and Fire Disasters 2001; XIV(4).
166. Kindhauser, MK. Global defence against the infectious disease threat. Communicable Diseases 2002. 2003; World Health Organization, Geneva.
167. World Health Organization (WHO). Quantifying selected major risks to health. The World Health Report 2002 (Chapter 4). 2002; World Health Organization, Geneva.
168. World Health Organization (WHO). Global water supply and sanitation assessment. 2000; World Health Organization, Geneva.
169. Grimwood K, Forbes DA. Acute and persistent diarrhea. Pediatric Clinics of North America 2009; 56(6):1343-61.
170. Carville KS, Lehmann D, Hall G, Moore H, et al. Infection is the major component of the disease burden in aboriginal and non-aboriginal Australian children: a population-based study. Pediatric Infectious Diseases Journal 2007; 26(3): 210-216.
171. Gracey M, Cullinane J. Gastroenteritis and environmental health among Aboriginal infants and children in Western Australia. Journal of Paediatrics and Child Health 2003; 39(6): 427-431.
172. Gracey M, Lee AH, Yau KK. Hospitalisation for gastroenteritis in Western Australia. Archives of Disease in Childhood 2004; 89(8): 768-772.
173. Moore HC, Manoharan KR, Lim FJ, Shellam G, et al. Diverging Trends in Gastroenteritis Hospitalizations during Two Decades in Western Australian Aboriginal and Non-Aboriginal Children. Pediatric Infectious Diseases Journal 2013; 32(11): 1169-74.
174. Power and Water Corporation. Drinking water quality summary report 2011. http://www.powerwater.com.au/_data/assets/pdf_file/0010/44785/2011_ies_water_summary.pdf.
175. Akpor OB, Muchie M. Remediation of heavy metals in drinking water and wastewater treatment systems: Processes and applications. International Journal of Physical Sciences 2010; 5(12): 1807-1817.
176. W.A. Government Committee of Inquiry, Education and Health Standing Committee, Legislative Assembly. Response of the Western Australian Government to the Western Australian in relation to the cause and extent of lead pollution in the Esperance Area. Perth, Government of Western Australia. 2007. http://www.esperanceport.com.au/downloads/ inquiry/LeadInquiry28Nov07.pdf.
177. Gulson B, Korsch M, Matisons M, Douglas C, et al. Windblown lead carbonate as the main source of lead in blood of children from a seaside community: an example of local birds as ‘‘canaries in the mine’’. Environmental Health Perspectives 2009; 117(1): 148-154.
178. W.A. Department of Health. Rainwater tank sample results current to 7 June 2007. 2007. http://www.public.health.wa.gov.au/cproot/1941/2/Rainwater%20tank%20results%20 June%202007.pdf.
179. Heyworth JS, Mullan N. Environmental lead and nickel contamination of tank rainwater in Esperance, Western Australia: an evaluation of the cleaning program. Journal of Water Resource and Protection 2009; 1(1): 1-9.
180. Gilbert SG, Weiss B. A rationale for lowering the blood lead action level from 10 to 2 microg/dL. Neurotoxicology 2006; 27(5): 693-701.
181. Patnaik P. A comprehensive guide to the hazardous properties of chemical substances, 3rd edn. Hoboken, NJ: John Wiley, 2007.
182. Townsend I. Don’t drink the water. ABC Radio National. Background Briefing, 31st March, 2013. http://www.abc.net.au/radionational/programs/backgroundbriefing/2013-03-31/ 4594752.
183. W.A. Department of Water. Remote drinking water sources – self-supplied Indigenous communities. Water Quality Protection Note 89. 2009. http://www.water.wa.gov.au/ PublicationStore/first/88087.pdf
184. Murdoch L. Polluted water leaking into Kakadu from uranium mine. The Age, Darwin. 2009. http://www.theage.com.au/national/polluted-water-leaking-into-kakadu-from-uranium-mine-20090312-8whw.html
185. Johansen MP, Twining JR. Radionuclide concentration ratios in Australian terrestrial wildlife and livestock: data compilation and analysis. Radiation and Environmental Biophysics 2010; 49(4): 603-11.
186. Bailie RS, Wayte KJ. Housing and health in Indigenous communities: key issues for housing and health improvement in remote Aboriginal and Torres Strait Islander communities. Australian Journal of Rural Health 2006; 14(5): 178-183.
187. Australian Bureau of Statistics. The health and welfare of Australia’s Aboriginal and Torres Strait Islander peoples, Oct 2010. Housing Circumstances: Overcrowding. 2011; Canberra: ABS.
188. McDonald EL. Closing the gap and Indigenous housing. Medical Journal of Australia 2011; 195(11-12): 652-653.
189. Beggs PJ, Siciliano F. Spatial relationship between dwelling crowding and selected causes of morbidity in Sydney, Australia, 1994-97. Australian Geographer 2001; 32(3): 377-401.
190. Zubrick SR, Silburn SR, Lawrence DM, Mitrou FG, et al. The social and emotional wellbeing of Aboriginal children and young people: vol 2. 2005. Perth: Telethon Institute for Child Health Research and Curtin University of Technology.
191. Menzies School of Health Research. Environmental health handbook: A practical manual for remote communities, Menzies School of Health Research, Northern Territory. 2000.
192. Howden-Chapman, P, Wilson, N. Housing and health. In: P Howden-Chapman and M Tobias (Eds); Social inequalities in health: New Zealand. Wellington: Ministry of Health, 1999; 133–145.
193. Australian Bureau of Statistics. The health and welfare of Australia’s Aboriginal and Torres Strait Islander peoples, 2008. Housing Circumstances: Housing and Health. 2008; Canberra: ABS.
194. Bailie RS, Si D, O’Donoghue L, Dowden M. Indigenous health: effective and sustainable health services through continuous quality improvement. Medical Journal of Australia 2007; 186(10): 525-527.
195. Leach AJ, Boswell JB, Asche V, Nienhuys TG, et al. Bacterial colonization of the nasopharynx predicts very early onset and persistence of otitis media in Australian Aboriginal infants. Pediatric Infectious Disease Journal 1994; 13: 983-989.
196. N.S.W. Department of Health. Closing the gap: 10 years of Housing for Health in N.S.W. An evaluation of a healthy housing intervention. 2010. http://www0.health.nsw. gov.au/ pubs/2010/pdf/housing_health_010210.pdf
197. Department of Families, Housing, Community Services and Indigenous Affairs. National partnership agreement on remote Indigenous housing. 2013. http://www. fahcsia.gov.au/our-responsibilities/indigenous-australians/programsservices/housing/ national-partnership-agreement-on-remote-indigenous-housing.